��Ŀ����
��8�֣����������NO2��NO�ȣ���������������γ����������꣬�������γɹ⻯ѧ��������˱���Ժ��е�������ķ������д�����
��1��������������Һ�����շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2 +NO+2NaOH�T2NaNO2+H2O ��2NO2 + 2NaOH�TNaNO2+ NaNO3 + H2O ��
�ڷ�Ӧ���У�����NaNO2��NԪ�صĻ��ϼ�Ϊ ���������뻹ԭ��������֮��Ϊ ���ڷ�Ӧ���У��������� ����ԭ���� ��
��2������β���к���һ��������һ����̼���������������ʶԴ�����Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�����Ӧ���������壬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3����������
������NH3��Ҳ��������������������磬���������������Ӧ��8NH3+6NO2�T7N2+12H2O��
��ij�����ų�������NO2����Ϊ0.5%�����������������1.0��103��3����״�������ַ�������Ҫ ǧ�˰��� ������������2λС������
��8�֣���1��+3 ��23:15 ���������� ���������� ��ÿ��1�֣�
��2��2 NO +2 CO  2 CO2 + N2��2�֣� ��3��5.06 ��2�֣�
2 CO2 + N2��2�֣� ��3��5.06 ��2�֣�
��������
�����������1�����ݻ�ѧʽ��֪������NaNO2��NԪ�صĻ��ϼ�Ϊ��3�ۡ�����NO2�е�Ԫ�صĻ��ϼ۴ӣ�4�۽��͵���3�ۡ�NO�е�Ԫ�صĻ��ϼ��Դӣ�2�����ߵ���3�ۣ�����������ͻ�ԭ�������ʵ���֮����1:1���ڷ�Ӧ����ֻ�е�Ԫ�صĻ��ϼ۷����仯������NO2����������Ҳ�ǻ�ԭ����
��2��˵��������Ӧ���ǵ�����CO2����Ӧ�Ļ�ѧ����ʽ��CO��NO������Ӧ���������壬2 NO +2 CO  2 CO2 + N2��
2 CO2 + N2��
��3��1.0��103��3����״�������ַ�����NO2�������1.0��103��3��0.5%��5m3��5000L�����Ը��ݷ�Ӧ�Ļ�ѧ����ʽ��֪����Ҫ�����������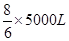 �����״���°�����������
�����״���°�����������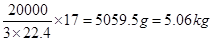 ��
��
���㣺����������ԭ��Ӧ���й��жϡ����㡢����ʽ����д
�������������е��Ѷȵ����⣬����ע�ػ���������������������ѵ��������������ѧ�������������������ѧ���������⡢��������������Ҳ����������ѧ���Ļ���������ʶ����ǿѧ����ѧϰ�����ԡ�

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�