��Ŀ����
�������ռ���������ij�������ԭ�ӣ��ó�����Ĵ��������պ��赲̫�����к���ǿ�������߷��䣬������Ϊ�Ĵ�����Ⱦ���ƻ������㣬�������CF2Cl2���ŷźͳ����ɻ�β��(NO2��NO)���ŷš������ƻ���ǿ����Ҫԭ���������ƻ������Ļ����ǰ���ʽ���еġ��磺A��CF2Cl2���������������²���Clԭ�ӣ��Գ�����������õ��ƻ����á���ͳ�ƣ�һ�����������ӿ����ƻ�������������ӣ����ѭ����������������ѧ����ʽ���벹���2����ѧ����ʽ��(1)CF2Cl2![]() Cl+CF2Cl
Cl+CF2Cl
(2)________________________________
(3)O+ClO![]() Cl+O2,�侻���Ϊ________________��
Cl+O2,�侻���Ϊ________________��
(д����ʽ)��������Ӧ��Cl��������_________________��
B��NO��NO2��O3��O�����ķ�ӦΪ��O3+NO![]() NO2+O2��_________________������ӦҲ����ѭ�����ܷ�Ӧ����ʽΪO+O3
NO2+O2��_________________������ӦҲ����ѭ�����ܷ�Ӧ����ʽΪO+O3![]() 2O2������NO���ƻ�������Ĺ���������______���ã�NO2Ϊ________��
2O2������NO���ƻ�������Ĺ���������______���ã�NO2Ϊ________��
O3+Cl![]() O2+ClO O+O3
O2+ClO O+O3![]() 2O2 ���� NO2+O
2O2 ���� NO2+O![]() NO+O2 �� ����
NO+O2 �� ����
������A.�����⣬ʹ�����ֽ����Clԭ�ӣ���ϵ��Ӧ(3)����(2)����ClʹO3���O2���Լ����ClO����O3+Cl![]() O2+ClO;��(2)(3)��Ӽ��þ������
O2+ClO;��(2)(3)��Ӽ��þ������
B��A��ȣ����������������ȥ����һ����Ӧ��������һ����Ӧ��

��ϰ��ϵ�д�
�����Ŀ
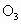 )����ԭ�ӣ��ó�����Ĵ��������պ��赲̫�������к���ǿ��������䣮������Ϊ�Ĵ�����Ⱦ����ƻ������㣬�糬���ٷɻ��ŷ����еĵ�������(NO��
)����ԭ�ӣ��ó�����Ĵ��������պ��赲̫�������к���ǿ��������䣮������Ϊ�Ĵ�����Ⱦ����ƻ������㣬�糬���ٷɻ��ŷ����еĵ�������(NO��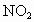 )�����Ǻ�
)�����Ǻ�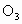 ��O�������·�Ӧ��
��O�������·�Ӧ��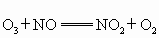 ��
��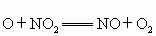 ����������Ӧ����ѭ��������˵����ȷ����
����������Ӧ����ѭ��������˵����ȷ����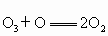
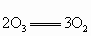
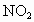 ����������
����������