��Ŀ����
 ��2013?�Ͳ���ģ����1��д��Co2+���ӻ�̬�ļ۵����Ų�ʽ��
��2013?�Ͳ���ģ����1��д��Co2+���ӻ�̬�ļ۵����Ų�ʽ��3d7
3d7
����2��SO32-�Ŀռ乹���ǣ�
������
������
����3��OCN-��CO2�ǵȵ����壬��OCN-��Cԭ�ӵ��ӻ���ʽ�ǣ�
sp
sp
����4������������BN��������кܸߵ��۵㣬Bԭ�Ӻ�Nԭ�Ӿ�Ϊsp2�ӻ����þ����д��ڵ��������У�
���ۼ������»���������Ӽ���������
���ۼ������»���������Ӽ���������
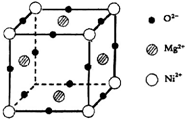
����5��Ԫ��O��Mg��Ni���γ�һ�־��壬�侧����ͼ��ʾ���ھ����У�ÿ��Ni2+������
8
8
��Mg2+������λ���þ���Ļ�ѧʽ��Mg2NiO3
Mg2NiO3
����������1��Co��27��Ԫ�أ�Co2+���Ӻ���3d����Ϊ��۵��ӣ�
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������������Ľṹ��ʯī���ƣ�����ʯī�Ľṹȷ���þ����д��ڵ���������
��5��ÿ��Ni2+���ӵ�Mg2+������λ��=2��8��
�����þ�̯��ȷ���仯ѧʽ��
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������������Ľṹ��ʯī���ƣ�����ʯī�Ľṹȷ���þ����д��ڵ���������
��5��ÿ��Ni2+���ӵ�Mg2+������λ��=2��8��
| 1 |
| 2 |
����⣺��1��Co��27��Ԫ�أ�Co2+���Ӻ�����25�����ӣ�3d����Ϊ��۵��ӣ�3d�ܼ�����7�����ӣ�������۵����Ų�ʽΪ3d7���ʴ�Ϊ��3d7��
��2��SO32-��Sԭ�Ӽ۲���Ӷ�=3+
(6+2-3��2)=4������һ���µ��Ӷԣ�������ռ乹���������ͣ��ʴ�Ϊ�������ͣ�
��3��OCN-��Cԭ�Ӽ۲���Ӷ�=2+
(4+1-1��2-1��3)=2�����Բ���sp�ӻ����ʴ�Ϊ��sp��
��4������������Ľṹ��ʯī���ƣ����侧��Ҳ�Ƿֲ�ṹ������Ƿ��Ӽ������������ڵ�Bԭ����Nԭ�Ӽ��Ǽ��Թ��ۼ����ʴ�Ϊ�����ۼ������»���������Ӽ�����������
��5��ÿ��Ni2+���ӵ�Mg2+������λ��=2��8��
=8���þ�����þ���Ӹ���=4��
=2�������Ӹ���=8��
=1�������Ӹ���=12��
=3�������仯ѧʽΪ��Mg2NiO3��
�ʴ�Ϊ��8��Mg2NiO3��
��2��SO32-��Sԭ�Ӽ۲���Ӷ�=3+
| 1 |
| 2 |
��3��OCN-��Cԭ�Ӽ۲���Ӷ�=2+
| 1 |
| 2 |
��4������������Ľṹ��ʯī���ƣ����侧��Ҳ�Ƿֲ�ṹ������Ƿ��Ӽ������������ڵ�Bԭ����Nԭ�Ӽ��Ǽ��Թ��ۼ����ʴ�Ϊ�����ۼ������»���������Ӽ�����������
��5��ÿ��Ni2+���ӵ�Mg2+������λ��=2��8��
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 8 |
| 1 |
| 4 |
�ʴ�Ϊ��8��Mg2NiO3��
���������⿼�����ʽṹ���ӻ���ʽ���жϡ��ռ乹�͵��жϡ���ѧʽ��ȷ������λ�����жϵ�֪ʶ�㶼��ѧϰ�ص㣬�ѵ��ǣ�4���⣬��ȷ����ṹ�ǽ����ؼ���������֪ʶǨ�Ƶķ������н���Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
�����Ŀ