��Ŀ����
���з�Ӧ�����ӷ���ʽ��ȷ����(����)��
| A��ϡ����������Ӧ��2Fe��6H��=2Fe3����3H2�� |
| B��2 mol��L��1��AlCl3��Һ��7 mol��L��1��NaOH��Һ��������Ȼ�ϣ�2Al3����7OH��=Al(OH)3����AlO2����2H2O |
| C��Ba(OH)2��Һ�м���������NaHSO4��Һ��Ba2����2OH����2H����SO42��=BaSO4����2H2O |
D��NaHCO3��ˮ�⣺HCO3����H2O CO32����H3O�� CO32����H3O�� |
��B
��ϡ����������Ӧ��������Fe3����Ӧ������Fe2����A����2 mol Al3����7 mol OH����Ӧ������1 mol Al(OH)3��1 mol AlO2����B��ȷ��Ba(OH)2��Һ�м���������NaHSO4��Һ�����ӷ���ʽӦΪBa2����OH����H����SO42��=BaSO4����H2O��C����NaHCO3��ˮ�ⷽ��ʽΪHCO3����H2O H2CO3��OH����D����
H2CO3��OH����D����
 H2CO3��OH����D����
H2CO3��OH����D����
��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ
 3Fe3����NO����3H2O
3Fe3����NO����3H2O Cl2��+H2��+2OH��
Cl2��+H2��+2OH��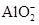 +2H2O
+2H2O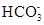 + OH-= CaCO3��+H2O
+ OH-= CaCO3��+H2O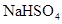 ��Һ��
��Һ�� ��Һ��ϣ�
��Һ��ϣ� ��
�� ��+
��+
 ��+
��+
 ��
��