��Ŀ����
11����ȫȼ��2.85g������A������8.8g������̼��4.5gˮ����1��A�ķ���ʽ��C8H18��
��2��B��A��һ����B��NaOH�Ĵ���Һ�м�����������ϩ��C1��C2��C2�ó�������ֻ������һ�ֻ�����D��D��������Ӧ����E��E��Ũ���Ṳ�Ⱥ�ֻ������һ��ϩ��F��F������ֻ�����ֻ�ѧ������ͬ����ԭ�ӣ�
��֪��R1��R4������ԭ�ӻ���������

�ش��������⣺
��F�Ľṹ��ʽ�ǣ�CH3��2C=CH2��
��E��Cu��Ag�������������º�O2��Ӧ�Ļ�ѧ����ʽ��2��CH3��2CHCH2OH+O2$��_{��}^{Cu��Ag}$2��CH3��2CHCHO+2H2O��
��Bת��ΪC1�Ļ�ѧ����ʽΪ��CH3��2CHCH2CHBrCH��CH3��2+NaOH$��_{��}^{�Ҵ�}$��CH3��2CHCH2CH=C��CH3��2+NaBr+H2O��
��C2��˳���칹�壬���з�ʽ�ṹ�Ľṹ��ʽ��
 ��
������
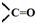 �ṹ��D��ͬ���칹����2�֣�
�ṹ��D��ͬ���칹����2�֣�
���� ��ȫȼ��2.85g������A������8.8g������̼��4.05gˮ��n��CO2��=$\frac{8.8g}{44g/mol}$=0.2mol��n��H2O��=$\frac{4.05g}{18g/mol}$=0.225mol������ԭ���غ�֪��A��C��Hԭ�Ӹ���֮��=0.2mol��0.45mol=4��9��0.2molC��0.45molH����֮��=0.2mol��12g/mol+0.45mol��1g/mol=2.85g������AΪ����
������ͨʽ֪��A����ʽΪC8H18��Ϊ����������
B��A��һ����B��NaOH�Ĵ���Һ�м�����������ϩ��C1��C2��C2�ó�������ֻ������һ�ֻ�����D��˵��C2Ϊ�Գƽṹ��D��������Ӧ����E��E��Ũ���Ṳ�Ⱥ�ֻ������һ��ϩ��F��˵��E�����Ӵ��ǻ���Cԭ������������ͬ����F������ֻ�����ֻ�ѧ������ͬ����ԭ�ӣ���F�ṹ��ʽΪ��CH3��2C=CH2��E�ṹ��ʽΪ��CH3��2CHCH2OH��DΪ��CH3��2CHCHO��C2Ϊ��CH3��2CHCH=CHCH��CH3��2��C1Ϊ��CH3��2C=CHCH2CH��CH3��2��B�ṹ��ʽΪ��CH3��2CHCH2CHBrCH��CH3��2��A�ṹ��ʽΪ��CH3��2CHCH2CH2CH��CH3��2���ݴ˷������
��� �⣺��ȫȼ��2.85g������A������8.8g������̼��4.05gˮ��n��CO2��=$\frac{8.8g}{44g/mol}$=0.2mol��n��H2O��=$\frac{4.05g}{18g/mol}$=0.225mol������ԭ���غ�֪��A��C��Hԭ�Ӹ���֮��=0.2mol��0.45mol=4��9��0.2molC��0.45molH����֮��=0.2mol��12g/mol+0.45mol��1g/mol=2.85g������AΪ����
������ͨʽ֪��A����ʽΪC8H18��Ϊ����������
B��A��һ����B��NaOH�Ĵ���Һ�м�����������ϩ��C1��C2��C2�ó�������ֻ������һ�ֻ�����D��˵��C2Ϊ�Գƽṹ��D��������Ӧ����E��E��Ũ���Ṳ�Ⱥ�ֻ������һ��ϩ��F��˵��E�����Ӵ��ǻ���Cԭ������������ͬ����F������ֻ�����ֻ�ѧ������ͬ����ԭ�ӣ���F�ṹ��ʽΪ��CH3��2C=CH2��E�ṹ��ʽΪ��CH3��2CHCH2OH��DΪ��CH3��2CHCHO��C2Ϊ��CH3��2CHCH=CHCH��CH3��2��C1Ϊ��CH3��2C=CHCH2CH��CH3��2��B�ṹ��ʽΪ��CH3��2CHCH2CHBrCH��CH3��2��A�ṹ��ʽΪ��CH3��2CHCH2CH2CH��CH3��2��
��1��ͨ�����Ϸ���֪��A����ʽΪC8H18���ʴ�Ϊ��C8H18��
��2����ͨ�����Ϸ���֪��F�ṹ��ʽΪ����CH3��2C=CH2���ʴ�Ϊ����CH3��2C=CH2��
��E�ṹ��ʽΪ��CH3��2CHCH2OH��E�ڴ��������·�������������ȩ����Ӧ����ʽΪ2��CH3��2CHCH2OH+O2$��_{��}^{Cu��Ag}$ 2��CH3��2CHCHO+2H2O��
�ʴ�Ϊ��2��CH3��2CHCH2OH+O2$��_{��}^{Cu��Ag}$ 2��CH3��2CHCHO+2H2O��
��C1Ϊ��CH3��2C=CHCH2CH��CH3��2��B������ȥ��Ӧ����C1����Ӧ����ʽΪ����CH3��2CHCH2CHBrCH��CH3��2+NaOH$��_{��}^{�Ҵ�}$��CH3��2CHCH2CH=C��CH3��2+NaBr+H2O��
�ʴ�Ϊ����CH3��2CHCH2CHBrCH��CH3��2+NaOH$��_{��}^{�Ҵ�}$��CH3��2CHCH2CH=C��CH3��2+NaBr+H2O��
��C2Ϊ��CH3��2CHCH=CHCH��CH3��2����˳���칹���䷴ʽ�칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��DΪ��CH3��2CHCHO����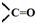 �ṹ��D��ͬ���칹����CH3CH2CH2CHO��CH3COCH2CH3���ֽṹ���ʴ�Ϊ��2��
�ṹ��D��ͬ���칹����CH3CH2CH2CHO��CH3COCH2CH3���ֽṹ���ʴ�Ϊ��2��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ���������F�ṹ��ʽΪͻ�ƿڲ�������˼ά���������ƶϣ����������л�������ż������ʹ�ϵ���ѵ���ͬ���칹�������жϣ�

| A�� | 18gD2O��18gH2O�к��е���������Ϊ10NA | |
| B�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | 1L 0.1mol•L-1��NaHCO3-��Һ��HCO3-��CO32-������֮��Ϊ0.1NA |
| A�� | ����ʱ���ɵ�������ȫ���������ӵĻ���������� | |
| B�� | CO2��ˮ��Һ���Ե��磬����CO2�ǵ���� | |
| C�� | �����ξ���������������Ӻ�������� | |
| D�� | ǿ����ʵ���Һ��������ǿ��������ʵ���Һ |
| A�� | ��ϩ��ʯ���ѽ��IJ��� | |
| B�� | ��Ȼ������Ҫ�Ļ���ԭ�� | |
| C�� | ʯ�͵ķ�����Եõ����͡�ú�͡����� | |
| D�� | ��������Ҫ������ú�ĸ�����ú���� |
| A�� | �屽û��ͬ���칹�� | B�� | �����������ӳɵõ������� | ||
| C�� | ����ױ�ֻ��һ�� | D�� | �ڶ��ױ�ֻ��һ�ֽṹ |
| A�� | �����ڿ�������ȼ�� | B�� | ̼��Ƹ��·ֽ� | ||
| C�� | ����������ˮ | D�� | ��˿��������ȼ�� |
| A�� | ƿ������Ļ���ɫ��dz | |
| B�� | ƿ�ڱ�����״Һ������ | |
| C�� | ������ֻ��CH3Cl��HCl | |
| D�� | �˷�Ӧ�������������CH3Cl��CH2Cl2��CHCl3��CCl4��HCl�Ļ���� |
 ��ͼ��ʾ��װ�ã�����һ�ֿɳ���ص�ʾ��ͼ��װ�ã���Ϊ���ص�ʾ��ͼ��װ�ã������ӽ���Ĥֻ����Na+ͨ������֪��س䡢�ŵ�Ļ�ѧ����ʽΪ2Na2S2+NaBr3 $?_{���}^{�ŵ�}$Na2S4+3NaBr�����պϿ���Kʱ��X��������Һ�ȱ��ɫ������˵������ȷ���ǣ�������
��ͼ��ʾ��װ�ã�����һ�ֿɳ���ص�ʾ��ͼ��װ�ã���Ϊ���ص�ʾ��ͼ��װ�ã������ӽ���Ĥֻ����Na+ͨ������֪��س䡢�ŵ�Ļ�ѧ����ʽΪ2Na2S2+NaBr3 $?_{���}^{�ŵ�}$Na2S4+3NaBr�����պϿ���Kʱ��X��������Һ�ȱ��ɫ������˵������ȷ���ǣ�������| A�� | �պ�Kʱ��װ�ã�����Na+���ҵ���ͨ�����ӽ���Ĥ | |
| B�� | �պ�Kʱ��A�缫�ĵ缫��ӦʽΪNaBr3+2Na++2e-�T3NaBr | |
| C�� | �պ�Kʱ��X�缫�ĵ缫��ӦʽΪ2Cl--2e-�TCl2�� | |
| D�� | �պ�Kʱ������0.1 mol Na+ͨ�����ӽ���Ĥ����X�缫�����������ڱ�״���µ����Ϊ1.12 L |
| A�� | ��Ba��OH��2��Һ�м��������NaHCO3��Һ��HCO3-+Ba2-+OH-�TBaCO3��+H2O | |
| B�� | ��С�մ���Һ�м������ʯ��ˮ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O | |
| C�� | ������������Һ�м����������������Һ��2HSO4-+2Ba2++2OH-=2BaSO4��+2H2O | |
| D�� | ��̼�������Һ�м������ʯ��ˮ��Ca2++HCO3-+OH-=CaCO3��+H2O |