��Ŀ����
��1��1mol H2O��1mol H2O2��Ƚϣ�������֮��Ϊ��_______________
��ԭ����֮��Ϊ��_________________; ����֮��Ϊ��________________��3�֣�
(2)��ʵ���ҳ���Ũ����Ͷ������̷�Ӧ����ȡ������������Ӧ�Ļ�ѧ����ʽΪ��
MnO2 + 4HCl(Ũ) MnCl2 + Cl2�� + 2H2O ��
MnCl2 + Cl2�� + 2H2O ��
ȡ8.7g��MnO2��50mL������Ũ���ᷢ����Ӧ��ȡ��������
�٣�����Cl2���ܽ⣩�����������ڱ�״���µ����Ϊ__________���μӷ�Ӧ��HCl��________mol����4�֣�
������Ӧ���ʣ����Һ�У���������AgNO3��Һ�����ɳ���57.4g����ԭŨ��������ʵ���Ũ�ȡ�(д���淶�Ľ������) (5��)
��ԭ����֮��Ϊ��_________________; ����֮��Ϊ��________________��3�֣�
(2)��ʵ���ҳ���Ũ����Ͷ������̷�Ӧ����ȡ������������Ӧ�Ļ�ѧ����ʽΪ��
MnO2 + 4HCl(Ũ)
 MnCl2 + Cl2�� + 2H2O ��
MnCl2 + Cl2�� + 2H2O ��ȡ8.7g��MnO2��50mL������Ũ���ᷢ����Ӧ��ȡ��������
�٣�����Cl2���ܽ⣩�����������ڱ�״���µ����Ϊ__________���μӷ�Ӧ��HCl��________mol����4�֣�
������Ӧ���ʣ����Һ�У���������AgNO3��Һ�����ɳ���57.4g����ԭŨ��������ʵ���Ũ�ȡ�(д���淶�Ľ������) (5��)
��1��1��1 1��2 9��17
��2����2.24L����λ��д���÷֣� 0.4
��n(AgCl)= 0.4mol��1�֣�
n(Cl2)= 0.1mol��1�֣�
n(HCl)��="0.4mol" +0.1mol��2 =0.6mol��1�֣�
c(HCl)=" 0.6mol/0.05L" =12mol/L��2�֣�
��2����2.24L����λ��д���÷֣� 0.4
��n(AgCl)= 0.4mol��1�֣�
n(Cl2)= 0.1mol��1�֣�
n(HCl)��="0.4mol" +0.1mol��2 =0.6mol��1�֣�
c(HCl)=" 0.6mol/0.05L" =12mol/L��2�֣�
��

��ϰ��ϵ�д�
 ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д�
�����Ŀ
 ��
��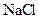 ��
��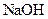 �Ļ����Һ�У����е�
�Ļ����Һ�У����е�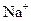 ��
��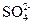 ��
��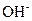 �ĸ�������8:1:2������Һ��
�ĸ�������8:1:2������Һ��