��Ŀ����
�������ӷ���ʽ��������ʵ��������ǣ� ��
| A����������Һ��ͨ������CO2��C6H5O��+CO2+H2O��C6H5OH+HCO3�� |
| B����̼��������Һ�м������������������Һ��Ba2��+OH��+HCO3��="=" BaCO3��+H2O |
| C�������Ƶ�������ͭ����Һ������ȩ�е�ȩ���� CH3CHO + 2Cu(OH)2 + OH��  CH3COO��+ Cu2O�� + 3H2O CH3COO��+ Cu2O�� + 3H2O |
| D����Ca(ClO)2��Һ��ͨ�����SO2��Ca2++ 2ClO�� +SO2��H2O=CaSO4����2H++ Cl��+ HClO |
D
������ƾ��������ԣ���SO2����������ԭ��Ӧ����ԭ�����������ӣ�����ѡ��D�Ǵ���ģ��������ȷ�ģ���ѡD��

��ϰ��ϵ�д�
 ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ
 Cl2��+H2��+2OH��
Cl2��+H2��+2OH�� CH2=CH2��+Br��+H2O
CH2=CH2��+Br��+H2O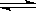 H3O++S2-
H3O++S2-