��Ŀ����
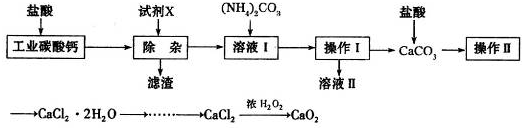
ʵ�����Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�Ϊԭ����ȡCaCl2��H2O��CaO2����Ҫ�������£�

��1�������Լ�X��������ҺpHΪ���Ի������Գ�ȥ��Һ��Al3+��Fe3+����������Ҫ�ɷ���___________���Լ�X����ѡ�����е�________�����ţ���
A.CaO B.CaCO3 C.NH3��H2O D.Ba(OH)2
��2������II�н�������Ũ��ʱ�������Ǽܡ��ƾ����⣬����Ҫ��������__________��
��3����CaCl2��ȡCaO2�ķ�Ӧ�У��¶Ȳ���̫�ߵ�ԭ����_______________��
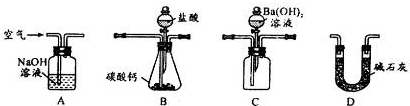
��4��������װ�òⶨ��ҵ̼��Ƶ���������

�ټ���װ��B���������õ�ʵ�������__________________________��
�ڰ�A��B��C��D˳�����ӣ�Ȼ���Aװ��ͨ�������Ŀ����_______________��
��װ��D������Ϊ______________________��
��ʵ��ʱ��ȷ��ȡ10.00g��ҵ̼���3�ݣ�����3�βⶨ�����BaCO3������ƽ������Ϊ17.73g������Ʒ��CaCO3����������Ϊ__________________��
����ȥע���⣬ÿ��2�֣���14�֣���1��Fe(OH)3��Al(OH)3 AC ��2������������������ǯ��
��3����ֹ�¶ȹ���H2O2�ֽ�
��4���ٹرշ�Һ©���Ļ�������ֹˮ�м�ס��ߣ����ұ��ã���Ƥ�ܴ������ұߣ���������ߣ������ܲ���ˮ�У���Bװ�ã����ֵ����������ݲ�������ȴ��������һ��Һ���γɣ�ע�����������ã��������ɣ� ���ž�װ���ж�����̼��1�֣�
�۷�ֹ������CO2��Ba(OH)2��Һ��Ӧ��1�֣� ��90%
��������
�����������1���ڼ��������£�Al3+��Fe3+�ֱ�����������������������������������������Ҫ�ɷ���Fe(OH)3��Al(OH)3������ʵ�����Թ�ҵ̼���Ϊԭ����ȡCaCl2��H2O��CaO2�ģ������Լ�X����ѡ��̼��ơ�����Ϊ���������µ����ʣ����Բ���ѡ����������������ѡ�������ơ����ں���ʵ���м���̼��泥�����X�������ǰ�ˮ������ѡAC��
��2�������Ȼ���������ˮ������Ҫ�õ��Ȼ��ƾ��壬Ӧ��������Ũ����ȴ�ᾧ�����Բ���II�н�������Ũ��ʱ�������Ǽܡ��ƾ����⣬����Ҫ������������������������ǯ����
��3����CaCl2��ȡCaO2�ķ�Ӧ�У���Ҫ˫��ˮ�μӡ�����˫��ˮ�ֽ⣬�����¶Ȳ���̫�ߵ�ԭ���ǣ���ֹ�¶ȹ���H2O2�ֽ⡣
��4��������Bװ���к��з�Һ©��������Ҫ�����������ԣ���ȷ�IJ���Ӧ���ǹرշ�Һ©���Ļ�������ֹˮ�м�ס��ߣ����ұ��ã���Ƥ�ܴ������ұߣ���������ߣ������ܲ���ˮ�У���Bװ�ã����ֵ����������ݲ�������ȴ��������һ��Һ���γɣ�ע�����������á�
��ʵ��ԭ�������������̼��Ʒ�Ӧ����CO2��CO2������������������̼�ᱵ������Ȼ��ͨ������Cװ�õļ��ɡ������ڷ�Ӧ��װ���л����CO2������ͨ�������Ŀ�����ž�װ���ж�����̼��
�����ڿ����к���CO2�����Dװ���м�ʯ�ҵ������Ƿ�ֹ������CO2��Ba(OH)2��Һ��Ӧ��
�ܸ���̼ԭ���غ��֪��CaCO3��������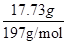 ��100g/mol��9.00g��������Ʒ��CaCO3����������Ϊ
��100g/mol��9.00g��������Ʒ��CaCO3����������Ϊ ��100%��90%��
��100%��90%��
���㣺�������ʵij��ӡ��Լ���������ѡ��Ӧ������֪��װ�������Լ��顢���ʺ����IJⶨ��