��Ŀ����
��2011?����ģ�⣩[��ѧ-ѡ���л���ѧ����]
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ����ͼ��ʾ������B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壮

��ش�
��1��D�ķ���ʽΪ
��2����ķ�Ӧ����Ϊ
a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д����Ӧ��Ļ�ѧ����ʽ
��4����˳���칹�ҽṹ����2��-CH3��A��ͬ���칹��Ľṹ��ʽΪ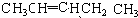
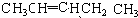 ��
��
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ
 ��
��
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ����ͼ��ʾ������B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壮

��ش�
��1��D�ķ���ʽΪ
C5H10O
C5H10O
B�����������ŵ�����Ϊ��ԭ��
��ԭ��
����2����ķ�Ӧ����Ϊ
d
d
��ķ�Ӧ����Ϊa��b
a��b
������ĸ��ţ���a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д����Ӧ��Ļ�ѧ����ʽ
CH3-CH��CH3��-CH2-CH2Br+NaOH
CH3-CH��CH3��-CH=CH2+NaBr+H2O
| �Ҵ� |
| �� |
CH3-CH��CH3��-CH2-CH2Br+NaOH
CH3-CH��CH3��-CH=CH2+NaBr+H2O
��| �Ҵ� |
| �� |
��4����˳���칹�ҽṹ����2��-CH3��A��ͬ���칹��Ľṹ��ʽΪ
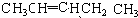
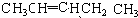
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ


������B����NaOH�Ҵ���Һ���ȵ������·�Ӧ����A����A�к���C=C���л���AΪ����������ͼ��������Է�������Ϊ70������
=5����A�ķ���ʽΪC5H10��B�ķ���ʽΪC5H11Br��D����������Һ��Ӧ��˵��D�к���-CHO��E�к���-COOH��B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬˵������4�ֲ�ͬ��Hԭ�ӣ�
DӦΪ CH3-CH��CH3��-CH2-CHO��EӦΪ CH3-CH��CH3��-CH2-COOH��CΪ CH3-CH��CH3��-CH2-CH2OH��BΪCH3-CH��CH3��-CH2-CH2Br����AΪCH3-CH��CH3��-CH=CH2������л���Ľṹ�����ʽ����⣮
| 70 |
| 14 |
DӦΪ CH3-CH��CH3��-CH2-CHO��EӦΪ CH3-CH��CH3��-CH2-COOH��CΪ CH3-CH��CH3��-CH2-CH2OH��BΪCH3-CH��CH3��-CH2-CH2Br����AΪCH3-CH��CH3��-CH=CH2������л���Ľṹ�����ʽ����⣮
����⣺B����NaOH�Ҵ���Һ���ȵ������·�Ӧ����A����A�к���C=C���л���AΪ����������ͼ��������Է�������Ϊ70������
=5����A�ķ���ʽΪC5H10��B�ķ���ʽΪC5H11Br��D����������Һ��Ӧ��˵��D�к���-CHO��E�к���-COOH��B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬˵������4�ֲ�ͬ��Hԭ�ӣ�
DӦΪ CH3-CH��CH3��-CH2-CHO��EӦΪ CH3-CH��CH3��-CH2-COOH��CΪ CH3-CH��CH3��-CH2-CH2OH��BΪCH3-CH��CH3��-CH2-CH2Br����AΪCH3-CH��CH3��-CH=CH2��
��1�������Ϸ�����֪DΪCH3-CH��CH3��-CH2-CHO������ʽΪC5H10O��BΪCH3-CH��CH3��-CH2-CH2Br�����еĹ�����Ϊ��ԭ�ӣ��ʴ�Ϊ��C5H10O����ԭ�ӣ�
��2����Ӧ��Ϊ��ȥ��Ӧ����Ӧ��Ϊ�ӳɷ�Ӧ���������ӳɷ�Ӧ���ɴ���ҲΪ��ԭ��Ӧ���ʴ�Ϊ��d��a��b��
��3����Ӧ��ΪCH3-CH��CH3��-CH2-CH2Br���Ҵ���������NaOH��Ӧ����ϩ����Ϊ��ȥ��Ӧ������ʽΪCH3-CH��CH3��-CH2-CH2Br+NaOH
CH3-CH��CH3��-CH=CH2+NaBr+H2O��
�ʴ�Ϊ��CH3-CH��CH3��-CH2-CH2Br+NaOH
CH3-CH��CH3��-CH=CH2+NaBr+H2O��
��4����˳���칹�ҽṹ����2��-CH3��A��ͬ���칹���У�C=C��Ӧ����������ͬ���������ṹ��ʽΪ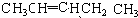 ��
��
�ʴ�Ϊ��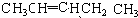 ��
��
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ��˵������-CHO��������������������������˵������-OH�����ܷ�����ȥ��Ӧ��˵��-OH��λ��Cԭ���ϲ���H������ܵĽṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| 70 |
| 14 |
DӦΪ CH3-CH��CH3��-CH2-CHO��EӦΪ CH3-CH��CH3��-CH2-COOH��CΪ CH3-CH��CH3��-CH2-CH2OH��BΪCH3-CH��CH3��-CH2-CH2Br����AΪCH3-CH��CH3��-CH=CH2��
��1�������Ϸ�����֪DΪCH3-CH��CH3��-CH2-CHO������ʽΪC5H10O��BΪCH3-CH��CH3��-CH2-CH2Br�����еĹ�����Ϊ��ԭ�ӣ��ʴ�Ϊ��C5H10O����ԭ�ӣ�
��2����Ӧ��Ϊ��ȥ��Ӧ����Ӧ��Ϊ�ӳɷ�Ӧ���������ӳɷ�Ӧ���ɴ���ҲΪ��ԭ��Ӧ���ʴ�Ϊ��d��a��b��
��3����Ӧ��ΪCH3-CH��CH3��-CH2-CH2Br���Ҵ���������NaOH��Ӧ����ϩ����Ϊ��ȥ��Ӧ������ʽΪCH3-CH��CH3��-CH2-CH2Br+NaOH
| �Ҵ� |
| �� |
�ʴ�Ϊ��CH3-CH��CH3��-CH2-CH2Br+NaOH
| �Ҵ� |
| �� |
��4����˳���칹�ҽṹ����2��-CH3��A��ͬ���칹���У�C=C��Ӧ����������ͬ���������ṹ��ʽΪ
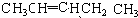 ��
���ʴ�Ϊ��
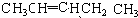 ��
����5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ��˵������-CHO��������������������������˵������-OH�����ܷ�����ȥ��Ӧ��˵��-OH��λ��Cԭ���ϲ���H������ܵĽṹ��ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�����������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע���������Ϣ����DΪ�������ͻ�ƿڽ����ƶϣ�ע������л�������ŵĽṹ�����ʣ�Ϊ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ