题目内容
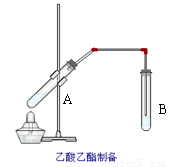
如图在试管A中先加入2mL的甲,并在摇动下缓缓加入2mL乙,充分摇匀,冷却后再加入丙,用玻璃棒充分搅拌后将试管固定在铁架台上,在试管B中加入5ml丁溶液,按图连接好装置,用酒精灯对试管A小火加热3~5min后,改用大火加热,当观察到B试管中有明显现象时停止实验。试回答: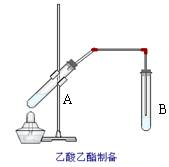
(1)写出下列物质的化学式(不用指出浓度)
甲 乙
丙 丁
(2)甲物质所含官能团的名称为
(3)写出试管A中发生反应的化学方程式
(4)若用18O标记乙醇中的氧元素,则18O会出现在哪种生成物中?
(5)试管甲中加入浓硫酸的主要目的是
(6)B中长导管不宜伸入试管的溶液中,原因是
(7)B中使用丁溶液的作用是
(1)CH3CH2OH,H2SO4,CH3COOH,Na2CO3
(2)羟基
(3)乙醇与乙酸的酯化反应(条件1分,可逆符号1分,其余2分,共4分)
(4)乙酸乙酯
(5)催化剂(多答不扣分)
(6)防倒吸
(7)反应乙酸,溶解乙醇,降低乙酸乙酯的溶解度 (此空3分)
(没有作说明的每空2分)
此答案仅供参考
解析

练习册系列答案
 培优口算题卡系列答案
培优口算题卡系列答案 开心口算题卡系列答案
开心口算题卡系列答案 口算题卡河北少年儿童出版社系列答案
口算题卡河北少年儿童出版社系列答案
相关题目