��Ŀ����
ij�л���A������������ܶ�Ϊ43��A����������Ʒ�Ӧ������ɫ���壬������̼���Ʒ�Ӧ������ɫ���壬������ʹ������Ȼ�̼��Һ��ɫ��A��ȫȼ��ֻ����CO2��H2O ��
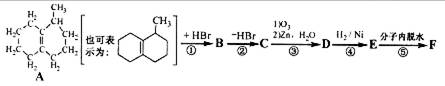
��1��A����Է�������Ϊ ��A�к��е�2�������ŷֱ��� �� ���ýṹ��ʽ��ʾ����
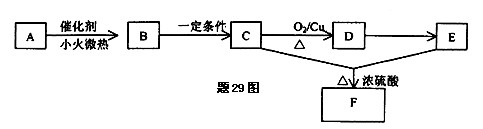
��2�����˴Ź����ⷢ��A��ͼ�����£�
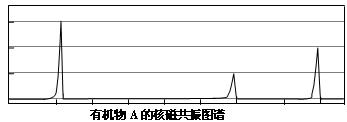
��д��A�Ľṹ��ʽ��________________________________________________��
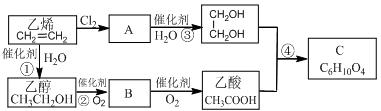
��3����д��A��״���Ӧ�����л���B�Ļ�ѧ��Ӧ����ʽ��
��
��4��B��һ�������¿��Է����ۺϷ�Ӧ�����л�������д����Ӧ�Ļ�ѧ����ʽ��
��
��5��B��ͬ���칹���ж��֣���д�����ֺ�Bͬ����ͬ���칹��Ľṹ��ʽ ��
��1��A����Է�������Ϊ ��A�к��е�2�������ŷֱ��� �� ���ýṹ��ʽ��ʾ����
��2�����˴Ź����ⷢ��A��ͼ�����£�

��д��A�Ľṹ��ʽ��________________________________________________��
��3����д��A��״���Ӧ�����л���B�Ļ�ѧ��Ӧ����ʽ��
��
��4��B��һ�������¿��Է����ۺϷ�Ӧ�����л�������д����Ӧ�Ļ�ѧ����ʽ��
��
��5��B��ͬ���칹���ж��֣���д�����ֺ�Bͬ����ͬ���칹��Ľṹ��ʽ ��
��1��M��86 -COOH 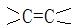
��2��CH2=C��CH3��COOH
��3��CH2=C��CH3��COOH+CH3OH CH2=C��CH3��COOCH3+H2O
CH2=C��CH3��COOCH3+H2O
��4��nCH2=C��CH3��COOCH3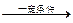
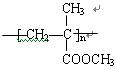
��5��CH3CH=CHCOOCH3 CH2=CHCH2COOCH3 CH2=CHCOOCH2CH3 ����2��
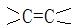
��2��CH2=C��CH3��COOH
��3��CH2=C��CH3��COOH+CH3OH
 CH2=C��CH3��COOCH3+H2O
CH2=C��CH3��COOCH3+H2O��4��nCH2=C��CH3��COOCH3
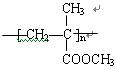
��5��CH3CH=CHCOOCH3 CH2=CHCH2COOCH3 CH2=CHCOOCH2CH3 ����2��
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
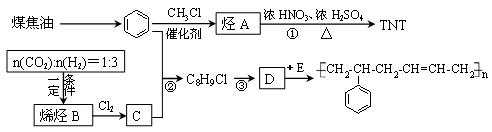

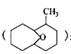 �⣬���õ�����ͬ���칹�壬
�⣬���õ�����ͬ���칹�壬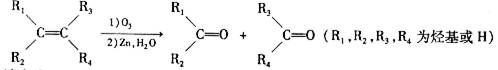

 �����������NaOH��Һ
�����������NaOH��Һ
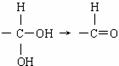 �����
���л��� ��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ졣�����йظ��л����˵������ȷ���ǣ� ��
��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ졣�����йظ��л����˵������ȷ���ǣ� ��