��Ŀ����
(10��)��1��������Aֻ��C��H��N����Ԫ�أ�������������Ϊ19.7%����Է�������������100����A�ķ���ʽΪ ����A�����к���Ԫ���ṹ�����ü���ʽ��ʾA�Ľṹʽ ��
��2��������ȡ�����ȴ��������棨�ṹ���Ʊ��������ۺ϶��ɣ���Ӧ���£�
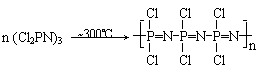 |
��д���ȴ���������Ľṹʽ
�������е�P-Cl����ˮ�����У����л�����ȡ��Cl������ˮ�����ȡ���ȼ�����ܷ�����кܴ���ƣ���д��������CF3-CH2-ONa��Ӧ�Ļ�ѧ��Ӧ����ʽ��
��
��3����������ͼ��ʾ�üױ���ȡ4-���������Ĺ��̣���������ɣ���
![]()
��1��C4H9N ��2�֣�![]() ��2�֣�
��2�֣�
��2����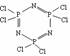 ��1�֣�
��1�֣�
�� ��2�֣�
��2�֣�
��3�� ��3�֣�
��3�֣�

(10��)���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
(1)�������ƽ�����ȷ���ǣ�
|
| ��ȶ��� | ���� |
| A | Cl2+H2O | I2+H2O |
| B | C+2CuO | C+SiO2 |
| C | Na2O+H2O == 2NaOH | CuO+H2O == Cu��OH��2 |
| D | Ca(ClO)2+CO2+H2O== CaCO3��+2HClO | Ca(ClO)2+SO2+H2O== CaSO3��+2HClO |
(2)��ѧϰ��±��Ԫ�صĸ������ʺ����±���ʾ�������ṩ��������Ԫ�صIJ�������.����Ԫ������������������⣺
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵�(��) | ��218.4 | 113 |
| 450 |
| ���ʷе�(��) | ��183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | ��2 | ��2����4����6 | ��2����4����6 |
|
| ԭ�Ӱ뾶 | ������ | |||
| ������H2 ��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
�������۵㷶Χ������_______ �����ڵĻ��ϼۿ�����_______ ��
���������ڵ��⻯��ˮ��Һ��������ǿ������˳���� (�ѧʽ)��
���������н�ǿ��________(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ___________________________________��
(10��)���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
(1)�������ƽ�����ȷ���ǣ�
| | ��ȶ��� | ���� |
| A | Cl2+H2O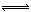 HCl+H HCl+H ClO ClO | I2+H2O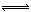 HI+HIO HI+HIO |
| B | C+2CuO 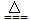 2Cu+CO2�� 2Cu+CO2�� | C+SiO2  Si+ CO2�� Si+ CO2�� |
| C | Na2O+H2O ="=" 2NaOH | CuO+H2O ="=" Cu��OH��2 |
| D | Ca(ClO)2+CO2+H2O="=" CaCO3��+2HClO | Ca(ClO)2+SO2+H2O="=" CaSO3��+2HClO |
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵�(��) | ��218.4 | 113 | | 450 |
| ���ʷе�(��) | ��183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | ��2 | ��2����4����6 | ��2����4����6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2 ��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
���������ڵ��⻯��ˮ��Һ��������ǿ������˳���� (�ѧʽ)��
���������н�ǿ��________(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ___________________________________��
(10��)���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
(1)�������ƽ�����ȷ���ǣ�
|
|
��ȶ��� |
���� |
|
A |
Cl2+H2O |
I2+H2O |
|
B |
C+2CuO |
C+SiO2 |
|
C |
Na2O+H2O == 2NaOH |
CuO+H2O == Cu��OH��2 |
|
D |
Ca(ClO)2+CO2+H2O== CaCO3��+2HClO |
Ca(ClO)2+SO2+H2O== CaSO3��+2HClO |
(2)��ѧϰ��±��Ԫ�صĸ������ʺ����±���ʾ�������ṩ��������Ԫ�صIJ�������.����Ԫ������������������⣺
|
Ԫ�� |
8O |
16S |
34Se |
52Te |
|
�����۵�(��) |
��218.4 |
113 |
|
450 |
|
���ʷе�(��) |
��183 |
444.6 |
685 |
1390 |
|
��Ҫ���ϼ� |
��2 |
��2����4����6 |
��2����4����6 |
|
|
ԭ�Ӱ뾶 |
������ |
|||
|
������H2 ��Ӧ��� |
��ȼʱ���� |
���Ȼ��� |
�����ѻ��� |
����ֱ�ӻ��� |
�������۵㷶Χ������_______ �����ڵĻ��ϼۿ�����_______ ��
���������ڵ��⻯��ˮ��Һ��������ǿ������˳���� (�ѧʽ)��
���������н�ǿ��________(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ___________________________________��
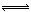 HCl+HClO
HCl+HClO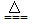 2Cu+CO2��
2Cu+CO2��