��Ŀ����
��10�֣�ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ�� A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ�����Т٢ڢ�����ͬ�ַ�Ӧ���͡�����ͼ�ش��������⣺
��1��д��A��B��C��D�Ľṹ��ʽ��
A__________________________________________��
B__________________________________________��
C__________________________________________��
D__________________________________________��
��2��д���ڡ��ܡ���������Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��________________________________________����Ӧ����________________��
��________________________________________����Ӧ����________________��
��________________________________________����Ӧ����________________��
��10�֣�ÿ��1�֣���1��CH2=CH2��CH3CH3��CH3CH2Cl��CH3CH2OH
��2����CH2=CH2��HCl CH3CH2Cl �ӳɷ�Ӧ
CH3CH2Cl �ӳɷ�Ӧ

�������������A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ������A����ϩ����ϩ�����к���̼̼˫���������������ӳɷ�Ӧ�������飬��B�����顣��ϩ���Ȼ���ӳ����������飬��C�������飬�������������ȡ����Ӧ��Ҳ�����������顣��ˮ�ӳ������Ҵ�����D���Ҵ�����ϩҲ�ܷ����Ӿ۷�Ӧ�����ɾ���ϩ����E�Ǿ���ϩ��
���㣺������ϩ�Ļ�ѧ���ʼ�����ʽ����д��
�����������ͻ�Ƶ���A��������ȷ������Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�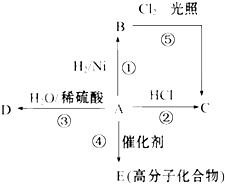 ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ�����Т٢ڢ�����ͬ�ַ�Ӧ���ͣ�
ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ�����Т٢ڢ�����ͬ�ַ�Ӧ���ͣ�



