��Ŀ����
�������Ũ�������ܷ����ۻ�����ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣Ϊ�ˣ��������������װ����֤�����������塣
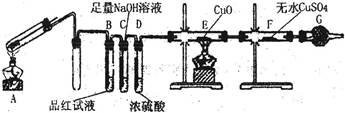
��1��֤����Ӧ����������������SO2���ɵ������� ��
��2��֤�������к���������ʵ�������� ��
��3��Ϊ�˽�һ��̽����Ӧ��A��Һ����Ԫ�صļ�̬�����ǽ��������µļ��裺
����1����Һ����Ԫ�ؼ���Fe3+Ҳ��Fe2+
����2����Һ����Ԫ��ֻ��Fe3+
����3����Һ����Ԫ��ֻ��________________
���ڼ���1�������Լ���0.01 mol/L����KMnO4��Һ��ϡ��ˮ��Һ��0.1 mal/L KI��Һ��
������Һ��KSCN��Һ������ˮ����̽����������Һ������ɱ������ݡ�
��ʵ��̽����

��1��֤����Ӧ����������������SO2���ɵ������� ��
��2��֤�������к���������ʵ�������� ��
��3��Ϊ�˽�һ��̽����Ӧ��A��Һ����Ԫ�صļ�̬�����ǽ��������µļ��裺
����1����Һ����Ԫ�ؼ���Fe3+Ҳ��Fe2+
����2����Һ����Ԫ��ֻ��Fe3+
����3����Һ����Ԫ��ֻ��________________
���ڼ���1�������Լ���0.01 mol/L����KMnO4��Һ��ϡ��ˮ��Һ��0.1 mal/L KI��Һ��
������Һ��KSCN��Һ������ˮ����̽����������Һ������ɱ������ݡ�
��ʵ��̽����
| ʵ����� | Ԥ������ | ���� |
| ȡ��Ӧ���A��Һ��װ��a��b���Թܣ�����٣���a�Թ��е��� �� | | |
| ����ڣ���b�Թ��е��� �� | | ��Һ����Fe3+ |
��16�֣���1��Ʒ����Һ��dz������ɫ�� ��2�֣�
��2��E�к�ɫ(CuO)��ɺ�ɫ��F�а�ɫ��ĩ(CuSO4)�����ɫ����2�֣�
��3������3����Һ����Ԫ��ֻ��Fe2+ ��2�֣�
��ʵ��̽����
��2��E�к�ɫ(CuO)��ɺ�ɫ��F�а�ɫ��ĩ(CuSO4)�����ɫ����2�֣�
��3������3����Һ����Ԫ��ֻ��Fe2+ ��2�֣�
��ʵ��̽����
| ʵ����� | Ԥ������ | ���� |
| ȡ��Ӧ���A��Һ��װ��a��b���Թܣ�����٣���a�Թ��е���������1�֣�0.01mol/L����KMnO4��Һ��1�֣��� | KMnO4��Һ���Ϻ�ɫ��ȥ�����dz����2�֣� | ��Һ����Fe2+��2�֣� |
| ����ڣ���b�Թ��е������Σ�1�֣�KSCN��Һ�������1�֣�[�Σ���������0.1mol/LKI��Һ�͵�����Һ��1�֣�] �� | ��Һ��ΪѪ��ɫ��2�֣� [����Һ��Ϊ��ɫ��2�֣�] | ��Һ����Fe3+ |
�����������1��SO2����Ư���ԡ����ԡ���ԭ�Ժ������ԣ���ͼ��֪����ʵ����Ʒ����Һ�����Ƿ���SO2���ɣ���Ʒ����Һ��ɫ���dz��˵��װ��A�з�Ӧ���������庬��SO2����2����������ǿ��ԭ�ԣ��ڼ�����������ʹ��ɫ������ͭ��ԭΪ��ɫ�ĵ���ͭ��H2������Ϊ��ʹ��ɫ����ˮCuSO4���������H2O����װ��E�к�ɫ�����Ϊ��ɫ��F�а�ɫ��ĩ��Ϊ��ɫ��˵��װ��A�зų��������к���H2����3������������Ũ����ۻ���������һ�����������ﱡĤ��������ǿ�����ԡ�ǿ���Ե�Ũ������Խ�������Ϊ���Σ��������ĵ��������Խ����λ�ԭΪ�����Σ���˷�Ӧ��A����Һ����Ԫ�ؿ��ܼ���Fe2+����Fe3+��Ҳ����ֻ��Fe3+��������ֻ��Fe2+��������֪����1������2����Ϣ�ƶϼ���3Ϊ��Һ����Ԫ��ֻ��Fe2+�����������仯��������ʿ�֪��Fe2+���л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��Fe3+���������ԣ��ܽ�KI��Һ��ԭΪ��ʹ������Һ������I2��Fe2+��KSCN��Һ����죬��Fe3+��KSCN��Һ��죬��������Fe3+����Һ�ʻ�ɫ������ˮ����ɫ��ͬ���ɴ˿���ѡ���ʵ����Լ����ʵ�鷽����֤A����Һ�м���Fe2+����Fe3+��

��ϰ��ϵ�д�
�����Ŀ





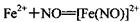 ����ɫ)����֪��ͬŨ�ȵ����������Ա�Fe3+��ǿ��
����ɫ)����֪��ͬŨ�ȵ����������Ա�Fe3+��ǿ��