��Ŀ����
��13�֣������ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺
��ȡWg���ᾧ�壬���100.00mLˮ��Һ
��1����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ���KMnO4������ɫΪֹ���������ķ�Ӧ
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
�Իش𣺣�1��ʵ���в���Ҫ�������У�����ţ�___________����ȱ�ٵ������У������ƣ�___________________________________________________��
a.������ƽ�������룬���ӣ�b.�ζ��� c.100mL��Ͳ d.100mL����ƿ e.�ձ� f.©�� g.��ƿ h.������ i.ҩ�� j.��ƿ
��2��ʵ���У���ҺKMnO4��ҺӦװ��_____ʽ�ζ����У���Ϊ____ ___________��
��3�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬������õ�xֵ��__________________________��ƫ��ƫС����Ӱ�죩
��4���ڵζ�����������amol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________________________mol��L-1���ɴ˿ɼ���x��ֵ��____________��
��ȡWg���ᾧ�壬���100.00mLˮ��Һ
��1����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ���KMnO4������ɫΪֹ���������ķ�Ӧ
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
�Իش𣺣�1��ʵ���в���Ҫ�������У�����ţ�___________����ȱ�ٵ������У������ƣ�___________________________________________________��
a.������ƽ�������룬���ӣ�b.�ζ��� c.100mL��Ͳ d.100mL����ƿ e.�ձ� f.©�� g.��ƿ h.������ i.ҩ�� j.��ƿ
��2��ʵ���У���ҺKMnO4��ҺӦװ��_____ʽ�ζ����У���Ϊ____ ___________��
��3�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬������õ�xֵ��__________________________��ƫ��ƫС����Ӱ�죩
��4���ڵζ�����������amol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________________________mol��L-1���ɴ˿ɼ���x��ֵ��____________��
��1��c , f , j ��ȱ������̨�����ζ��ܼУ�����ͷ�ιܣ�����ʱ�ã�
��2������ʽ�ζ��ܣ���KMnO4��Һ��ǿ�����ԣ��ܸ�ʴ��Ƥ�ܡ�
��3����Ӱ�졣����ȡ�Ĵ���Һ�����ʵ���һ����
��4��
��2������ʽ�ζ��ܣ���KMnO4��Һ��ǿ�����ԣ��ܸ�ʴ��Ƥ�ܡ�
��3����Ӱ�졣����ȡ�Ĵ���Һ�����ʵ���һ����
��4��

��

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�
�����Ŀ

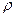 )����6mol/L HCl��Һ�У�����Ӧ����ͻȻ����ʱ������ȡ����Ƭ����ϴ��ɺ����������m2����п�Ʋ���Ϊ
)����6mol/L HCl��Һ�У�����Ӧ����ͻȻ����ʱ������ȡ����Ƭ����ϴ��ɺ����������m2����п�Ʋ���Ϊ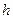 =
=