��Ŀ����
ȫ��ˮ����IJ����ḻ��Լռ�������ܲ�����99%�������С�����Ԫ�ء�֮�ƣ���ˮ���庬��Ϊ65mg/L���乤ҵ��ȡ�������Ƚ�����ͨ�뵽���������ӵĺ�ˮ�У������û�����������ȡ�����壮��ȡ���������¼��֣���1�����������������շ����÷������ÿ������崵�����ô�����Һ���գ�����������ữ�����ɵõ��嵥�ʣ��÷����漰���ķ�Ӧ�У���______��д�����ӷ���ʽ������
 ����
����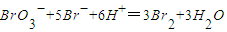 �����Т��з�����ԭ��Ӧ��������______��
�����Т��з�����ԭ��Ӧ��������______����2����������SO2���շ����÷����ǽ��崵������SO2��ˮ��Һ�����գ�ʹ��ת��Ϊ�����ᣬȻ�������������������ἴ�õ������壮д��������������ˮ��Һ��Ӧ�Ļ�ѧ����ʽ______��
��3���ܼ���ȡ�����÷��������õ�������ˮ�к��л��ܼ��е��ܽ�Ȳ�ͬ��ԭ��������ʵ�飮ʵ��������ȡ�õ�����Ҫʵ������������______�����п������ڵ��������ȡ�Լ���______������ţ���
���Ҵ� �����Ȼ�̼ ������ �ܱ���
���𰸡���������1��������ǿ�����ԣ������������������嵥�ʣ��������õ��ӻ��ϼ۽��Ͷ�������ԭ��Ӧ��
��2�����������ԣ����������л�ԭ�ԣ���ˮ��Һ�����������巢��������ԭ��Ӧ��
��3��������ȡʵ��������Ƿ�Һ©������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ԭ�ܼ������ܣ���ȡ�������ʲ���Ӧ��
����⣺��1��������ǿ�����ԣ������������������嵥�ʣ���������ԭ���������ӣ����ӷ�Ӧ����ʽΪ��
Cl2+2Br-=Br2+2Cl-��BrO3-+5Br-+6H+=3Br2+3H2O��BrO3-��Br�Ļ��ϼ���+5�۱�Ϊ0�ۣ�����BrO3-��������������ԭ��Ӧ���ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��BrO3-��
��2�����������ԣ����������л�ԭ�ԣ���ˮ��Һ�����������巢��������ԭ��Ӧ��������������ᣬ��Ӧ����ʽΪ��Br2+SO2+2H2O=2HBr+H2SO4���ʴ�Ϊ��Br2+SO2+2H2O=2HBr+H2SO4��
��3��������ȡʵ��������Ƿ�Һ©������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ԭ�ܼ������ܣ���ȡ�������ʲ���Ӧ��������ȡ����ѡȡ��֪���Ҵ������ᶼ��ˮ���ܣ����Բ�����������������ȡ���������Ȼ�̼�ͱ���
�ʴ�Ϊ����Һ©�����ڢܣ�
���������⿼����������ԭ��Ӧ����ȡ��֪ʶ�㣬����Ԫ�صĻ��ϼ۱仯����ȡ����ѡȡ����������ɣ��ѶȲ���
��2�����������ԣ����������л�ԭ�ԣ���ˮ��Һ�����������巢��������ԭ��Ӧ��
��3��������ȡʵ��������Ƿ�Һ©������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ԭ�ܼ������ܣ���ȡ�������ʲ���Ӧ��
����⣺��1��������ǿ�����ԣ������������������嵥�ʣ���������ԭ���������ӣ����ӷ�Ӧ����ʽΪ��
Cl2+2Br-=Br2+2Cl-��BrO3-+5Br-+6H+=3Br2+3H2O��BrO3-��Br�Ļ��ϼ���+5�۱�Ϊ0�ۣ�����BrO3-��������������ԭ��Ӧ���ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��BrO3-��
��2�����������ԣ����������л�ԭ�ԣ���ˮ��Һ�����������巢��������ԭ��Ӧ��������������ᣬ��Ӧ����ʽΪ��Br2+SO2+2H2O=2HBr+H2SO4���ʴ�Ϊ��Br2+SO2+2H2O=2HBr+H2SO4��
��3��������ȡʵ��������Ƿ�Һ©������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ԭ�ܼ������ܣ���ȡ�������ʲ���Ӧ��������ȡ����ѡȡ��֪���Ҵ������ᶼ��ˮ���ܣ����Բ�����������������ȡ���������Ȼ�̼�ͱ���
�ʴ�Ϊ����Һ©�����ڢܣ�
���������⿼����������ԭ��Ӧ����ȡ��֪ʶ�㣬����Ԫ�صĻ��ϼ۱仯����ȡ����ѡȡ����������ɣ��ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ