��Ŀ����
��1��773K���̶�����������У���ӦCO��g����2H2��g��
 CH3OH��g�������� �����仯����ͼ�����ߢ��ʾʹ�ô���ʱ�������仯����Ͷ��amol CO��2amol H2��ƽ��ʱ������0��1a mol CH3OH����Ӧ�;߹�ҵӦ�ü�ֵ��
CH3OH��g�������� �����仯����ͼ�����ߢ��ʾʹ�ô���ʱ�������仯����Ͷ��amol CO��2amol H2��ƽ��ʱ������0��1a mol CH3OH����Ӧ�;߹�ҵӦ�ü�ֵ�� 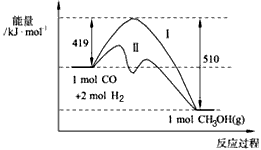
���������ݻ������ǰ���£������H2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��������ɣ�______________��
______________����
������������йص�˵���У���ȷ����______________ ������ĸ��ţ�����
a��ʹ�ô�����ʹ��ӦCO(g)��2H2��g��
 CH3OH��g�� ��H>��91 kJ��mol-1 ��
CH3OH��g�� ��H>��91 kJ��mol-1 �� b��ʹ�ô������ܹ���߷�Ӧ��ת����
c��ʹ�ô��������ܸı䷴Ӧ��ƽ�ⳣ��K
��2��������������Դ���������й㷺��;���ҹ�ѧ�����������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4�ķ�����װ������ͼI��ʾ��
��Fe�缫��____________�������������������
��Ni�缫�ĵ缫��ӦʽΪ��_________________��

��3��������ԭ��Ӧ��ʵ���ϰ��������ͻ�ԭ�������̡�������HNO3������һ����ԭ���̵ķ�Ӧʽ��
NO3-��4H+��3e-��NO��2H2O
�� KMnO4��Na2CO3��CuO��KI���������е�______________(�ѧʽ)��ʹ������ԭ���̷�����
��������ͼIIװ��ͨ���ⶨ�����������������ᱻ��ԭ�����ʣ�����Ӧ���Ũ�ȡ�����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ_________________________���㷴Ӧ���ʡ�
��2����������2H++2e-==H2����2H2O+2e-==H2+2OH-��
��3����λʱ�����ռ���������������ռ�һ��������������õ�ʱ�䣩

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�Ӧ�û�ѧ��Ӧ��Ҫ�о���ѧ��Ӧ���������Ⱥ����ʡ�
��1��773 K���̶������������,��ӦCO��g����2H2��g�� CH3OH��g��������
CH3OH��g��������
�����仯����ͼ�����ߢ��ʾʹ�ô���ʱ�������仯����Ͷ��amol CO��2amol H2��ƽ��ʱ������0.1a mol CH3OH����Ӧ�;߹�ҵӦ�ü�ֵ��

�� ��������Ͷ�ϱ�ʹ�÷�Ӧ���й�ҵӦ�ü�ֵ��CO��ƽ��ת������СΪ�� ��
�� ���������ݻ������ǰ���£������H2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��������ɣ� ���������� ��
�� �� ������������йص�˵���У���ȷ������ ������ĸ��ţ���
��
a��ʹ�ô�����ʹ��Ӧ CO(g)��2H2��g�� CH3OH��g�� ��H>��91
kJ��mol��1
CH3OH��g�� ��H>��91
kJ��mol��1
�� b��ʹ�ô������ܹ���߷�Ӧ��ת����
c��ʹ�ô��������ܸı䷴Ӧ��ƽ�ⳣ��K
��2��������������Դ���������й㷺��;���ҹ�ѧ�����������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4�ķ�����װ������ͼI��ʾ��
��Fe�缫���� �������������������
��Ni�缫�ĵ缫��ӦʽΪ������ ��
��3��������ͼIIװ��ͨ���ⶨ�����������������ᱻ��ԭ�����ʣ�����Ӧ���Ũ�ȡ� ����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ
����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ
���㷴Ӧ���ʡ�


 ����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ
����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ 
 ����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ
����������Ӱ�����ʵ�����ȷ��֮����ͨ���ⶨ  CH3OH(g)�����������仯����ͼ�����ߢ��ʾʹ�ô���ʱ�������仯����Ͷ��a
mol CO��2a mol H2��ƽ��ʱ������0.1a mol CH3OH����Ӧ�;߹�ҵӦ�ü�ֵ��
CH3OH(g)�����������仯����ͼ�����ߢ��ʾʹ�ô���ʱ�������仯����Ͷ��a
mol CO��2a mol H2��ƽ��ʱ������0.1a mol CH3OH����Ӧ�;߹�ҵӦ�ü�ֵ��

