��Ŀ����
��2013?��̨ģ�⣩��ʵ�����У�Ϊ��֤C12��Fe3+��SO2��������˳��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�еļ���װ����ʡ�ԣ��������Ѿ����飩��
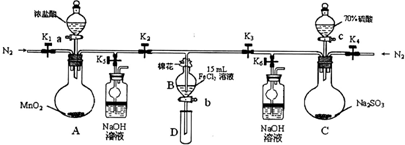
��1����K1��K4���ر�K5��K6��ͨ��һ��ʱ��N2��Ŀ����
��2���ر�K1��K3��K4������a���μ�һ������Ũ���ᣬ��A���ȣ�A�з�����Ӧ�����ӷ���ʽΪ
��3����B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2����K5����������ԭ����
��4������b��ʹԼ2mL����Һ�����Թ�D�У�����
��5�����ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3����K6����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����
��6�������Թ�D������b��ʹԼ2mL����Һ�����Թ�D�У�����B��Һ�е����ӣ�����SO42-������Լ���
A��Ba��NO3��2��Һ B��BaC12��Һ C��AgNO3��Һ D��Ba��OH��2��Һ
�ס��ҡ�����λͬѧ�ֱ������B�еõ�����Һ�������й����ӵļ�⣬���ǵļ����һ���ܹ�֤��������C12��Fe3+��SO2����
��װ���У����н�����Һ��

��1����K1��K4���ر�K5��K6��ͨ��һ��ʱ��N2��Ŀ����
�ų�װ���е�����
�ų�װ���е�����
����2���ر�K1��K3��K4������a���μ�һ������Ũ���ᣬ��A���ȣ�A�з�����Ӧ�����ӷ���ʽΪ
MnO2+4H++2Cl-
Mn2++Cl2��+H2O
| ||
MnO2+4H++2Cl-
Mn2++Cl2��+H2O
��
| ||
��3����B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2����K5����������ԭ����
���ն������������ֹ����ѹǿ���������Σ��
���ն������������ֹ����ѹǿ���������Σ��
����4������b��ʹԼ2mL����Һ�����Թ�D�У�����
KSCN��Һ
KSCN��Һ
����֤�������Ƿ���Fe3+���ӣ���5�����ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3����K6����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����
70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ�
70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ�
����6�������Թ�D������b��ʹԼ2mL����Һ�����Թ�D�У�����B��Һ�е����ӣ�����SO42-������Լ���
B
B
��A��Ba��NO3��2��Һ B��BaC12��Һ C��AgNO3��Һ D��Ba��OH��2��Һ
�ס��ҡ�����λͬѧ�ֱ������B�еõ�����Һ�������й����ӵļ�⣬���ǵļ����һ���ܹ�֤��������C12��Fe3+��SO2����
�ҡ���
�ҡ���
��| ��һ��B����Һ�������� | �ڶ���B����Һ�������� | |
| �� | ��Fe2+����Fe3+ | ��SO 42- |
| �� | ����Fe3+������Fe2+ | ��SO 42- |
| �� | ��Fe3+����Fe2+ | ��Fe2+ |
NaOH��Һ
NaOH��Һ
�������������ն�����������������ֹ��Ⱦ����
���ն�����������������ֹ��Ⱦ����
����������1��ͨ��һ��ʱ��N2���ų�װ���е�������
��2��A�з�������������Ũ����ķ�Ӧ�������Ȼ��̡�������ˮ��
��3���н����ɼ�K2����K5�����ն������������ֹ����ѹǿ������Σ�գ�
��4����������KSCN��Һ��ΪѪ��ɫ��
��5��������Ũ��Խ��Ӧ����Խ�죻
��6����Һ�����ԣ��������������Ӧѡ���Ȼ�����Һ�����е�һ�Σ�˵���������㣬���������Դ��������ӣ��ڶ�������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ���������е�һ����Fe3+����Fe2+���������������Դ��������ӣ��ڶ������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ����������н���NaOH��Һ������������������ֹ��Ⱦ������
��2��A�з�������������Ũ����ķ�Ӧ�������Ȼ��̡�������ˮ��
��3���н����ɼ�K2����K5�����ն������������ֹ����ѹǿ������Σ�գ�
��4����������KSCN��Һ��ΪѪ��ɫ��
��5��������Ũ��Խ��Ӧ����Խ�죻
��6����Һ�����ԣ��������������Ӧѡ���Ȼ�����Һ�����е�һ�Σ�˵���������㣬���������Դ��������ӣ��ڶ�������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ���������е�һ����Fe3+����Fe2+���������������Դ��������ӣ��ڶ������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ����������н���NaOH��Һ������������������ֹ��Ⱦ������
����⣺��1����K1��K4���ر�K5��K6��ͨ��һ��ʱ��N2��Ŀ�����ų�װ���е��������ʴ�Ϊ���ų�װ���е�������
��2��A�з�������������Ũ����ķ�Ӧ�������Ȼ��̡�������ˮ���÷�ӦΪMnO2+4H++2Cl-
Mn2++Cl2��+H2O���ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+H2O��
��3����B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2����K5��Ŀ�������ն������������ֹ����ѹǿ���������Σ�գ�
�ʴ�Ϊ�����ն������������ֹ����ѹǿ���������Σ�գ�
��4���������Ȼ�������Ӧ���������ӣ���������KSCN��Һ��ΪѪ��ɫ�������KSCN��Һ����֤�������Ƿ���Fe3+���ӣ��ʴ�Ϊ��KSCN��Һ��
��5����������Ũ��Խ��Ӧ����Խ�죬��70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ����Է�Ӧ���ʿ죬
�ʴ�Ϊ��70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ�
��6����Һ�����ԣ��������������Ӧѡ���Ȼ�����Һ�����е�һ�Σ�˵���������㣬���������Դ��������ӣ��ڶ�������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ���������е�һ����Fe3+����Fe2+���������������Դ��������ӣ��ڶ������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ����������н���NaOH��Һ�����ն�����������������ֹ��Ⱦ������
�ʴ�Ϊ��B���ҡ�����NaOH��Һ�����ն�����������������ֹ��Ⱦ������
��2��A�з�������������Ũ����ķ�Ӧ�������Ȼ��̡�������ˮ���÷�ӦΪMnO2+4H++2Cl-
| ||
| ||
��3����B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2����K5��Ŀ�������ն������������ֹ����ѹǿ���������Σ�գ�
�ʴ�Ϊ�����ն������������ֹ����ѹǿ���������Σ�գ�
��4���������Ȼ�������Ӧ���������ӣ���������KSCN��Һ��ΪѪ��ɫ�������KSCN��Һ����֤�������Ƿ���Fe3+���ӣ��ʴ�Ϊ��KSCN��Һ��
��5����������Ũ��Խ��Ӧ����Խ�죬��70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ����Է�Ӧ���ʿ죬
�ʴ�Ϊ��70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ�
��6����Һ�����ԣ��������������Ӧѡ���Ȼ�����Һ�����е�һ�Σ�˵���������㣬���������Դ��������ӣ��ڶ�������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ���������е�һ����Fe3+����Fe2+���������������Դ��������ӣ��ڶ������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ����������н���NaOH��Һ�����ն�����������������ֹ��Ⱦ������
�ʴ�Ϊ��B���ҡ�����NaOH��Һ�����ն�����������������ֹ��Ⱦ������
���������⿼������ʵ�鷽������Ƽ�������ԭ��Ӧ����ȷװ�õ����ü������ķ�Ӧ�ǽ��Ĺؼ���ע�������ԵıȽ��ǽ����ѵ㣬��Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ