��Ŀ����
���ʼҿ�ѧԺ2007��10��10���������������ŵ������ѧ������¹�����ѧ�ḥ���һ�����о����ĸ����?���ض����ڣ��Ա������ڹ�����滯ѧ�����о��������������Գɾ͡������?���ض�����Ҫ����֮һ�ǶԹ���һ��ʩ���ϰ���Ӧ���������о��������?���ض�������ij�¶��ºϳɰ���Ӧ�����ĸ�����Ӧ�������仯��ͼ��ʾ��ͼ�е�������λΪkJ?mol��1����ע��ͼ�С�����ʾ�ڴ��������������
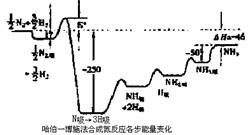
��ش��������⣺
��1���ںϳɰ���Ӧ�У�������Ӧ���ʵ�����Ҫ�IJ����� ������ĸ��
A�������ӽ���Ϊ��ԭ��
B����ԭ�ӵĽ�������
C������ӽ���Ϊ��ԭ��
D�����Ľ�������
��2���ϳɰ���Ӧ�Ļ���Ϊ���û�ѧ����ʽ��ʾ��
�� ��
��N2 ![]() N2��
N2�� ![]() 2N����
2N����
�� ��
��NH��+H�� ![]() NH3��
NH3��
��NH2��+H��![]() NH3��
NH3�� ![]() NH3
NH3
��3��ij�¶��ºϳɰ���Ӧ���Ȼ�ѧ����ʽΪ ��
��4��ij�¶��£��ϳɰ���ӦKe=3.0��103��mol?L��1����2�����¶��£�������㶨Ϊ10L���ĸ��ܱ������зֱ���룻��A��10mol��30mol H2��20molNH3��B��10molN2��30molH2��C��20molN2��60molH2��D��10molN2��28molH2��E��0.1molN2��0.3molH2��20MolNH3����Ӧ�ﵽƽ���N2��ת���������� ������ĸ����
��5���ϳɰ���ҵ���������õĦ��DFe��������Ҫ�ɷ���FeO��Fe2O3����������Fe3+��Fe3+�����ʵ���֮��Ϊ1��2ʱ�������������ߣ���Fe2O3Ϊԭ���Ʊ������������������м�������̿�ۡ��������·�Ӧ��2Fe2O3+C=4FeO+CO2��Ϊ�Ƶø��ֻ�����ߵĴ�����Ӧ��480gFe2O3��ĩ����̿�۵�����Ϊ g.
��1��B
��2����H2 ![]() 2H�� ��N��+H��
2H�� ��N��+H��![]() NH��
NH��
��3��N2(g)+3H2(g) ![]() 2NH3(g) ��H=92kJ?mol
2NH3(g) ��H=92kJ?mol
��4��C
��5��6

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�