��Ŀ����
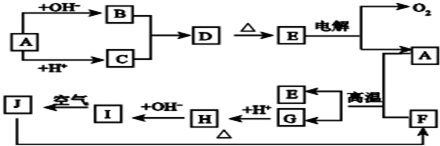
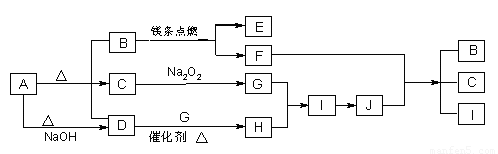
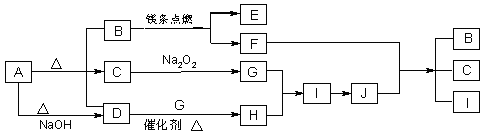
��ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����
����д���¿հף�
��1��A�Ļ�ѧʽ_________B�ĵ���ʽ_____________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��G��H________________________
F+J��B+C+I___________________________
��3��0��3mol I������C��Ӧת�Ƶ��ӵ����ʵ���Ϊ________mol
��4���ݻ�Ϊ10mL���Թ��г���I��G�Ļ�����壬������ʢˮ��ˮ���У�ˮȫ�������Թܣ���ԭ���������I��G������ֱ�Ϊ_____mL��_____mL��
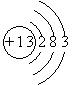
��1��NH4HCO3��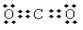
��2��4NH3+5O2 4NO+6H2O��C+4HNO3(Ũ)
4NO+6H2O��C+4HNO3(Ũ) CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��3��0.2
��4��82
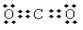
��2��4NH3+5O2
 4NO+6H2O��C+4HNO3(Ũ)
4NO+6H2O��C+4HNO3(Ũ) CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O��3��0.2
��4��82

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ