��Ŀ����
ʳƷ��ȫ��ϵ�������ƣ�Ӱ��ʳƷ��ȫ�����غ࣮ܶ
��1����ƫ������ϩ��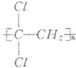 �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵģ�
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵģ�
��2������ֲ�����е�������[CH3��CH2��4-CH=CH-CH2-CH=CH-��CH2��7COOH]�����ܵͣ����й����������˵���У���ȷ���� ��
A������ʽΪC18H34O2 B��һ��������������ͣ�������������������Ӧ
C���ܺ�NaOH��Һ��Ӧ D����ʹ����KMnO4��Һ��ɫ
��3���پ��м״���CH3OH���������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ ��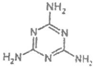
��4������¹�̷��¼�����Ԫ��--�����谷�ķ��ӽṹ��ʽ��ͼ�����������谷��˵����ȷ���� ��
A�������谷�����ʵ����� B�������谷�е�Ԫ�صĺ������ڵ������е��ĺ���
C�������谷��һ�������¿��ܻ������������ӳɷ�Ӧ D�������谷���ڰ�����
��5���ڵ����м�������Ƶõķ�˿�ж����������յ�ˮ������������ǣ������ʵ��
֤�������Ѿ�ȫ��ˮ�⣬д������������ͽ��� ��
��1����ƫ������ϩ��
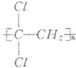 �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ���������2������ֲ�����е�������[CH3��CH2��4-CH=CH-CH2-CH=CH-��CH2��7COOH]�����ܵͣ����й����������˵���У���ȷ����
A������ʽΪC18H34O2 B��һ��������������ͣ�������������������Ӧ
C���ܺ�NaOH��Һ��Ӧ D����ʹ����KMnO4��Һ��ɫ
��3���پ��м״���CH3OH���������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ

��4������¹�̷��¼�����Ԫ��--�����谷�ķ��ӽṹ��ʽ��ͼ�����������谷��˵����ȷ����
A�������谷�����ʵ����� B�������谷�е�Ԫ�صĺ������ڵ������е��ĺ���
C�������谷��һ�������¿��ܻ������������ӳɷ�Ӧ D�������谷���ڰ�����
��5���ڵ����м�������Ƶõķ�˿�ж����������յ�ˮ������������ǣ������ʵ��
֤�������Ѿ�ȫ��ˮ�⣬д������������ͽ���
��������1���ɸ߷��ӽṹ��ʽ��֪��Ϊ�Ӿ۲�������к�̼̼˫����
��2�������Ậ̼̼˫�����Ȼ������ϩ������������ʷ�����
��3��Na�봼��Ӧ����������
��4���ɷ���ʽ��֪����N���ߣ���C=N���������ᣬֻ��������
��5���������յ�ˮ������������ǣ������Ѿ�ȫ��ˮ�⣬���õⵥ�ʼ�����۲����ڣ�
��2�������Ậ̼̼˫�����Ȼ������ϩ������������ʷ�����
��3��Na�봼��Ӧ����������
��4���ɷ���ʽ��֪����N���ߣ���C=N���������ᣬֻ��������
��5���������յ�ˮ������������ǣ������Ѿ�ȫ��ˮ�⣬���õⵥ�ʼ�����۲����ڣ�
����⣺��1���ɸ߷��ӽṹ��ʽ��֪��Ϊ�Ӿ۲�������к�̼̼˫�������ƫ������ϩ��CCl2=CH2�����Ӿ۷�Ӧ���ɣ��ʴ�Ϊ��CCl2=CH2��
��2�������Ậ̼̼˫�����Ȼ���
A���ɽṹ��ʽ��֪������ʽΪC18H32O2����A����
B����-COOH����һ��������������ͣ�������������������Ӧ����B��ȷ��
C����-COOH���ܺ�NaOH��Һ��Ӧ����C��ȷ��
D����̼̼˫������ʹ����KMnO4��Һ��ɫ����D��ȷ��
�ʴ�Ϊ��BCD��
��3��Na�봼��Ӧ������������Na��״��ķ�ӦΪ2CH3OH+2Na=2CH3ONa+H2�����ʴ�Ϊ��2CH3OH+2Na=2CH3ONa+H2����
��4��A�������谷�в����ļ������ǵ����ʣ���A����
B���ɷ���ʽ��֪����N���ߣ������谷�е�Ԫ�صĺ������ڵ������е��ĺ�������B��ȷ��
C����C=N�������谷��һ�������¿��ܻ������������ӳɷ�Ӧ����C��ȷ��
D�������谷�У��������ᣬֻ�������������ڰ����ᣬ��D����
�ʴ�Ϊ��BC��
��5���������յ�ˮ������������ǣ���֤�������Ѿ�ȫ��ˮ�⣬��ȡ��������ˮ������Һ�����м����ˮ������Һ������ɫ֤�������Ѿ���ȫˮ�⣬
�ʴ�Ϊ��ȡ��������ˮ������Һ�����м����ˮ������Һ������ɫ֤�������Ѿ���ȫˮ�⣮
��2�������Ậ̼̼˫�����Ȼ���
A���ɽṹ��ʽ��֪������ʽΪC18H32O2����A����
B����-COOH����һ��������������ͣ�������������������Ӧ����B��ȷ��
C����-COOH���ܺ�NaOH��Һ��Ӧ����C��ȷ��
D����̼̼˫������ʹ����KMnO4��Һ��ɫ����D��ȷ��
�ʴ�Ϊ��BCD��
��3��Na�봼��Ӧ������������Na��״��ķ�ӦΪ2CH3OH+2Na=2CH3ONa+H2�����ʴ�Ϊ��2CH3OH+2Na=2CH3ONa+H2����
��4��A�������谷�в����ļ������ǵ����ʣ���A����
B���ɷ���ʽ��֪����N���ߣ������谷�е�Ԫ�صĺ������ڵ������е��ĺ�������B��ȷ��
C����C=N�������谷��һ�������¿��ܻ������������ӳɷ�Ӧ����C��ȷ��
D�������谷�У��������ᣬֻ�������������ڰ����ᣬ��D����
�ʴ�Ϊ��BC��
��5���������յ�ˮ������������ǣ���֤�������Ѿ�ȫ��ˮ�⣬��ȡ��������ˮ������Һ�����м����ˮ������Һ������ɫ֤�������Ѿ���ȫˮ�⣬
�ʴ�Ϊ��ȡ��������ˮ������Һ�����м����ˮ������Һ������ɫ֤�������Ѿ���ȫˮ�⣮
���������⿼���л���Ľṹ�����ʣ�Ϊ�߿��������ͣ������л����������������ϵ��ע��֪ʶ��Ǩ��Ӧ�ã��������ᡢϩ�����������۵����ʼ��ɽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��12�֣���ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺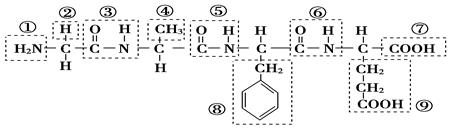
��1���û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�
��2��д���û�����ˮ�����ɵ�����һ�ְ�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
�� ʳƷ��ȫ��ϵ�Ź������ƣ�Ӱ��ʳƷ��ȫ�����غܶࡣ
��3����ƫ������ϩ(?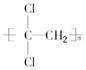 ?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
(4)����ֲ�����е�������[CH3(CH2)4��CH===CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����________��������ţ�
| A������ʽΪC18H34O2 |
| B��һ���������������(������)����������Ӧ |
| C���ܺ�NaOH��Һ��Ӧ |
| D����ʹ����KMnO4��Һ��ɫ |
��6���������յ�ˮ������������ǡ������ʵ��֤�������Ѿ�ȫ��ˮ�⣬д������������ͽ��ۣ�______________��

 ?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ� �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵġ�
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵġ�