��Ŀ����
����������д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1����NA��ʾ�����ӵ���������C2H2(��̬)��ȫȼ������CO2��Һ̬ˮ�ķ�Ӧ�У�ÿ��4NA������ת��ʱ���ų�450 kJ�����������Ȼ�ѧ����ʽΪ______________________��
��2����֪��1 mol H��H����1 mol N��H����1 mol N��N���ֱ���Ҫ��������436 kJ��395 kJ��940 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ____________________________��
��3���ѣ�Ti������Ϊ��������֮��ĵ�����������֪�ɽ��ʯ��TiO2����ȡ����Ti���漰�IJ���Ϊ��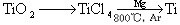
��֪����C(s)+O2(g)
 CO2(g); ��H=��395.5 kJ��mol-1
CO2(g); ��H=��395.5 kJ��mol-1
��2CO(g)+O2(g)  2CO2(g); ��H=��560 kJ��mol-1
2CO2(g); ��H=��560 kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s)  TiCl4(s)+2CO(g)�Ħ�H=�D80kJ/mol
TiCl4(s)+2CO(g)�Ħ�H=�D80kJ/mol
��TiO2(s)��Cl2(g)��Ӧ���Ȼ�ѧ����ʽΪ ��
��13�֣�(1) C2H2(g)+ 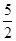 O2(g)��2CO2(g)+
H2O(l) ��H����1125 kJ/mol ��4�֣�
O2(g)��2CO2(g)+
H2O(l) ��H����1125 kJ/mol ��4�֣�
(2) N2(g)+ 3H2(g) 2NH3(g) ��H����122 kJ/mol ��4�֣�
2NH3(g) ��H����122 kJ/mol ��4�֣�
(3)TiO2(s)+ 2Cl2(g)��TiCl4(s)+ O2(g) ��H����151 kJ/mol ��5�֣�
��������
�����������1����Ȳ������̼Ԫ�صĻ��ϼ��ǣ�1�ۣ���Ӧ���Ϊ��4�ۣ�ʧȥ5�����ӣ���1mol��Ȳʧȥ10mol���ӣ���ÿ��4NA������ת��ʱ��������Ȳ�����ʵ�����0.4mol������ÿ����1mol��Ȳ�ų���������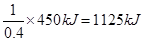 ����˸÷�Ӧ���Ȼ�ѧ����ʽ��C2H2(g)+
����˸÷�Ӧ���Ȼ�ѧ����ʽ��C2H2(g)+
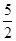 O2(g)��2CO2(g)+
H2O(l) ��H����1125 kJ/mol��
O2(g)��2CO2(g)+
H2O(l) ��H����1125 kJ/mol��
��2����Ӧ�Ⱦ��Ƕϼ����յ����������γɻ�ѧ�����ų��������IJ�ֵ������ݼ��ܿ�֪��ÿ����2mol�����ķ�Ӧ�ȡ�H��436kJ/mol��3��940kJ/mol��2��3��395kJ/mol����122 kJ/mol������Ӧ���Ȼ�ѧ����ʽ��N2(g)+ 3H2(g) 2NH3(g) ��H����122 kJ/mol��
2NH3(g) ��H����122 kJ/mol��
��3�����ݸ�˹���ɿ�֪���ۣ��ڣ��١�2�����õ���ӦTiO2(s)+ 2Cl2(g)��TiCl4(s)+ O2(g) �����Ը÷�Ӧ�ķ�Ӧ�Ȧ�H���D80kJ/mol��560 kJ/mol��395.5 kJ/mol��2����151 kJ/mol��
���㣺�����Ȼ�ѧ����ʽ����д�Լ���Ӧ�ȵ��йؼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�������߿�����Ҫ�ǿ���ѧ�����Ȼ�ѧ����ʽ��д����Ӧ�ȼ����Լ���˹���ɵ���Ϥ�˽�̶ȣ�ּ������ѧ�����������ɺ��ܽ����������������������ѧ���Ĺ淶����������
