��Ŀ����
�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���ĸ�һ������������������Ӳ�8�����ӡ��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ������������ˮ�������ԵĻ�����Y��Z��0.1mol/L��Y��ҺpH��1�����ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������LҲ����Z��ˮ��Һ��Ӧ������N�����������ɻ�����M����ش��������⣺
��1�������ӵĽṹʾ��ͼΪ ��
��2��д���ɼ�����Ԫ���γɵĻ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ������ʵĽṹʽ ��������������ڼ��������¿ɹ���ȼ�ϵ�أ��õ�طŵ�ʱ�������ķ�Ϊ ��
��3����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2��4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ ��
��4��д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ ��
��5������ͼ���M�ı�����Һ:

д���õ����з�����Ӧ���ܷ�Ӧ����ʽ ������ֵ���������Һ��μ��뵽��̪��Һ�У��۲쵽�������� ��
��1�������ӵĽṹʾ��ͼΪ ��
��2��д���ɼ�����Ԫ���γɵĻ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ������ʵĽṹʽ ��������������ڼ��������¿ɹ���ȼ�ϵ�أ��õ�طŵ�ʱ�������ķ�Ϊ ��
��3����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2��4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ ��
��4��д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ ��
��5������ͼ���M�ı�����Һ:

д���õ����з�����Ӧ���ܷ�Ӧ����ʽ ������ֵ���������Һ��μ��뵽��̪��Һ�У��۲쵽�������� ��
��1��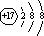
��2��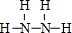 N2H4+4OH--4e-=N2��+2H2O
N2H4+4OH--4e-=N2��+2H2O
��3��2��3
��4��AlO2-+H++H2O�TAl(OH)3��
��5��2NaCl+H2O NaClO+H2�� ��Һ������ɫ
NaClO+H2�� ��Һ������ɫ
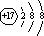
��2��
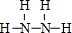 N2H4+4OH--4e-=N2��+2H2O
N2H4+4OH--4e-=N2��+2H2O��3��2��3
��4��AlO2-+H++H2O�TAl(OH)3��
��5��2NaCl+H2O
 NaClO+H2�� ��Һ������ɫ
NaClO+H2�� ��Һ������ɫ�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�أ��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������XΪNH3����ΪH����ΪN���ס�������ͬһ���壬���ԭ��������֪����ΪNa����ĸ�һ������������������Ӳ�8�����ӣ���ĸ�һ�������Ӻ��������Ϊ18������ΪCl�������백����Ӧ���ɵ�����ͬʱ������������ˮ�������ԵĻ�����Y��Z��0.1mol/L��Y��ҺpH��1����YΪNH4Cl��ZΪHCl�����ĵ��ʼ��������Na��Ԫ������������ˮ�������Һ��Ӧ������L��Ҳ����Z��HCl����ˮ��Һ��Ӧ������N����Ϊ���Խ�������ΪAlԪ�أ���LΪNaAlO2����������ɻ�����MΪNaCl����ʯī����������������������Ȼ���ˮ��Һ�������������������������ƣ��������������Ʒ�Ӧ�����Ȼ�����������ơ�
��1��Cl-�Ľṹʾ��ͼΪ��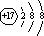 ��
��
��2��H��N��Ԫ���γɵĻ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ�������ΪN2H4����ṹʽΪ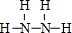 ��������Ӧ������Ӧ��N2H4�ڸ���ʧȥ���ӣ��������������ɵ�����ˮ�������缫��ӦʽΪN2H4+4OH--4e-=N2��+2H2O��
��������Ӧ������Ӧ��N2H4�ڸ���ʧȥ���ӣ��������������ɵ�����ˮ�������缫��ӦʽΪN2H4+4OH--4e-=N2��+2H2O��
��3�������백����Ӧ���ɵ�NH4Cl��HCl�����ʵ���֮��Ϊ2��4����NH3��Cl2��Ӧ�Ļ�ѧ����ʽ��4NH3+3Cl2�TN2+2NH4Cl+4HCl���ڷ�Ӧ�а�������ԭ�����������������������������ʰ���ֻռ��Ӧ����һ�룬�����������ʰ����뱻��ԭ�������������ʵ���֮��=2��3��
��4��������������������NaAlO2��Һ�з�����Ӧ�����ӷ���ʽΪ:AlO2-+H++H2O�TAl(OH)3����
��5����ⱥ���Ȼ�����Һ����Ӧ�ķ���ʽΪ��2NaCl+2H2O 2NaOH+Cl2��+H2�� ,
2NaOH+Cl2��+H2�� ,
ͬʱ������ӦCl2+2NaOH=NaCl+NaClO+H2O���ʸõ����з�����Ӧ���ܷ�Ӧ����ʽΪNaCl+H2O NaClO+H2��������õ�NaClO��Һ���Լ��ԣ��Ҿ���ǿ�����ԣ������̪��Һ�У��۲쵽��Һ������ɫ��
NaClO+H2��������õ�NaClO��Һ���Լ��ԣ��Ҿ���ǿ�����ԣ������̪��Һ�У��۲쵽��Һ������ɫ��
��1��Cl-�Ľṹʾ��ͼΪ��
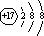 ��
����2��H��N��Ԫ���γɵĻ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ�������ΪN2H4����ṹʽΪ
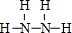 ��������Ӧ������Ӧ��N2H4�ڸ���ʧȥ���ӣ��������������ɵ�����ˮ�������缫��ӦʽΪN2H4+4OH--4e-=N2��+2H2O��
��������Ӧ������Ӧ��N2H4�ڸ���ʧȥ���ӣ��������������ɵ�����ˮ�������缫��ӦʽΪN2H4+4OH--4e-=N2��+2H2O����3�������백����Ӧ���ɵ�NH4Cl��HCl�����ʵ���֮��Ϊ2��4����NH3��Cl2��Ӧ�Ļ�ѧ����ʽ��4NH3+3Cl2�TN2+2NH4Cl+4HCl���ڷ�Ӧ�а�������ԭ�����������������������������ʰ���ֻռ��Ӧ����һ�룬�����������ʰ����뱻��ԭ�������������ʵ���֮��=2��3��
��4��������������������NaAlO2��Һ�з�����Ӧ�����ӷ���ʽΪ:AlO2-+H++H2O�TAl(OH)3����
��5����ⱥ���Ȼ�����Һ����Ӧ�ķ���ʽΪ��2NaCl+2H2O
 2NaOH+Cl2��+H2�� ,
2NaOH+Cl2��+H2�� ,ͬʱ������ӦCl2+2NaOH=NaCl+NaClO+H2O���ʸõ����з�����Ӧ���ܷ�Ӧ����ʽΪNaCl+H2O
 NaClO+H2��������õ�NaClO��Һ���Լ��ԣ��Ҿ���ǿ�����ԣ������̪��Һ�У��۲쵽��Һ������ɫ��
NaClO+H2��������õ�NaClO��Һ���Լ��ԣ��Ҿ���ǿ�����ԣ������̪��Һ�У��۲쵽��Һ������ɫ��
��ϰ��ϵ�д�
�����Ŀ

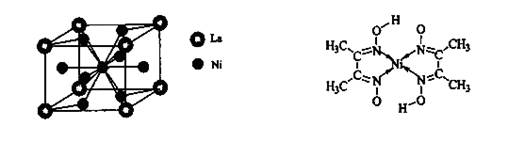
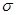 ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��