��Ŀ����
��6�֣�ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϡ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
��1�����������������ԭ��
��2����һ��˵������Ի�����ɵ�Σ����
��3������ij��ѧ����С�������������������д�ʩ������Ϊ���в�����
��4��������ʯ�����������᳧�ų������Է�ˮ������ԭ���Ļ�ѧ����ʽ��
��5��Ũ����Ū�����Ϻ�Ӧ������ˮ��ϴ��Ȼ��Ϳ��̼�����ơ�����ϡ����Ū�����ϣ�
�����Ҫ������Ҫ������������������
��1�����������������ԭ��
��2����һ��˵������Ի�����ɵ�Σ����
��3������ij��ѧ����С�������������������д�ʩ������Ϊ���в�����
| A�������᳧������� | B�����黷�������������� |
| C�������᳧�ų��ķ����е�SO2�������ŷ� | D�������;�����ý�����ȼ�� |
��5��Ũ����Ū�����Ϻ�Ӧ������ˮ��ϴ��Ȼ��Ϳ��̼�����ơ�����ϡ����Ū�����ϣ�
�����Ҫ������Ҫ������������������
��1���ŷŶ��������������ú��ȼ�ϣ�1�֣�
��2���ữ��������ʴ���ƻ�ɭ��ֲ���ʴ�������һ�㼴�ɣ���1�֣�
��3��A��1�֣�
��4��Ca(OH)2+H2SO4=CaSO4+2H2O����ѧʽ1�֣���ƽ1�֣�
��5����Ҫ��1�֣� ϡ�����е�ˮ���������Ũ���ᣨ1�֣�
��2���ữ��������ʴ���ƻ�ɭ��ֲ���ʴ�������һ�㼴�ɣ���1�֣�
��3��A��1�֣�
��4��Ca(OH)2+H2SO4=CaSO4+2H2O����ѧʽ1�֣���ƽ1�֣�
��5����Ҫ��1�֣� ϡ�����е�ˮ���������Ũ���ᣨ1�֣�
��1��SO2�ܺ�ˮ��Ӧ���������ᣬ�����������ԣ��Ӷ�������ꡣ
��2������Ի�����ɵ�Σ����Ҫ���ữ��������ʴ���ƻ�ɭ��ֲ���ʴ�����
��3�������᳧��������ǽ������İ취������ѡ��A�Dz��ģ���ѡA��
(4)���������Ǽ�ܺ����ᷴӦ������ʽΪCa(OH)2+H2SO4=CaSO4+2H2O��
��5������ϡ�����е�ˮ������Ҳ���Ũ���ᣬ��������Ҫ�ġ�
��2������Ի�����ɵ�Σ����Ҫ���ữ��������ʴ���ƻ�ɭ��ֲ���ʴ�����
��3�������᳧��������ǽ������İ취������ѡ��A�Dz��ģ���ѡA��
(4)���������Ǽ�ܺ����ᷴӦ������ʽΪCa(OH)2+H2SO4=CaSO4+2H2O��
��5������ϡ�����е�ˮ������Ҳ���Ũ���ᣬ��������Ҫ�ġ�

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
�����Ŀ
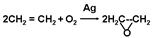 ��������ɫ��ѧ��������ġ�ԭ�Ӿ��á�
��������ɫ��ѧ��������ġ�ԭ�Ӿ��á�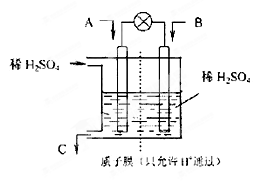
 n(H+)= mol��
n(H+)= mol�� N2O4(g) ��H<0��ƽ�ⳣ��K=13.3��
N2O4(g) ��H<0��ƽ�ⳣ��K=13.3�� CH3OH(g)����֪�÷�Ӧ��300OCʱ�Ļ�ѧƽ�ⳣ��Ϊ0.27�����¶��½�2moI CO��3mol H2��2molCH3OH(g)�����ݻ�Ϊ2 L���ܱ������У���ʱ��Ӧ�� ��(�������Ӧ������С��������淴Ӧ������С�����ƽ��״̬��)��
CH3OH(g)����֪�÷�Ӧ��300OCʱ�Ļ�ѧƽ�ⳣ��Ϊ0.27�����¶��½�2moI CO��3mol H2��2molCH3OH(g)�����ݻ�Ϊ2 L���ܱ������У���ʱ��Ӧ�� ��(�������Ӧ������С��������淴Ӧ������С�����ƽ��״̬��)��