��Ŀ����
��9�֣����Ż�����ʶ��ǿ�������ԴԽ��Խ�ܵ����ǹ�ע��
��1��������һ������Ľྻȼ�ϡ���֪��
CH4��g��+2O2��g��= CO2��g��+2H2O��g������H= ��802��3kJ��mol-1
H2O��1�� =H2O��g������H =+44��0kJ��mol-l
��4��8g����������ȫ��ȼ������Һ̬ˮ���ų�����Ϊ ��
��2�����ü�����ˮ��Ӧ�Ʊ���������ԭ�����ۣ������ƹ��ֵ��
�÷�ӦΪCH4��g��+H2O��g�� CO��g��+3H2��g��;��H=+206��lkJ��mol-l��
CO��g��+3H2��g��;��H=+206��lkJ��mol-l��
����800��ʱ����Ӧ�Ļ�ѧƽ�ⳣ��K=l��0��ijʱ�̲�ø��¶����ܱ������и����ʵ����ʵ���Ũ�����±���
���ʱ�����淴Ӧ���ʵĹ�ϵ�� �������ţ�
| A��v������>v���棩 | B��v������<v���棩 |
| C��v������=v���棩 | D�����ж� |
����t= ��P= ��
���ʵ��2��3��Ŀ����
ʵ��l��2��3�з�Ӧ�Ļ�ѧƽ�ⳣ���Ĵ�С��ϵ�� ����K1��K2��K3��ʾ����

�������㣺��ѧƽ��ļ��㣻�йط�Ӧ�ȵļ��㣻�����Դ��
��������1�����ݸ�˹����д������������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ���ٸ��ݷ�Ӧ�ȼ��㣻
��2���ټ������ʱ��ʱ��Ũ���̣���ƽ�ⳣ����ȣ��жϷ�Ӧ���з��ݴ˽��
�ڲ�ȡ���Ʊ���������������Ա�ʵ���Ҫ�������ж�T��P��ֵ��
��ȡ���Ʊ��������Ա�ʵ��2��3��������ͬ������ʵ��Ŀ�ģ�
ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬���¶����ߣ�ƽ�������ȷ����ƶ�����������Ӧ�����ƶ���ƽ�ⳣ������
��𣺽⣺��1����֪��Ӧ����CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-802.3kJ?mol-1��
��H2O��1��=H2O��g����H=+44.0kJ?mol-1��
��-�ڡ�2�ã�����������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1��
����4.8g����������ȫȼ������Һ̬ˮ���ų�����Ϊ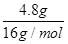 ��890.3kJ?mol-1=267.1kJ��
��890.3kJ?mol-1=267.1kJ��
�ʴ�Ϊ��267.1kJ��
��2���ٴ�ʱ��Ũ����Ϊ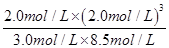 =0.63��С��ƽ�ⳣ��k=1�����Է�Ӧ������Ӧ���У���v��������v���棩��
=0.63��С��ƽ�ⳣ��k=1�����Է�Ӧ������Ӧ���У���v��������v���棩��
�ʴ�Ϊ��A��
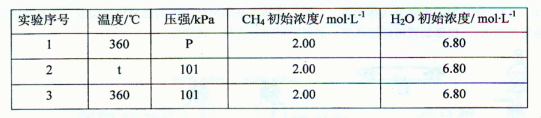
������ʵ������Ҫ���¶�Ϊ360���480�桢ѹǿΪ101kPa��303kPa����ȡ���Ʊ�������̽���¶ȡ�ѹǿ�Ի�ѧ��Ӧ���ʵ�Ӱ�죬�ɱ���֪tΪ480��pΪ303��
�Ա�ʵ��2��3��ֻ���¶Ȳ�ͬ������������ͬ������ʵ��2��3��Ŀ����̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죻
ʵ��1��3�¶���ͬ��ƽ�ⳣ����ͬ����K1=K3���Ƚ�ʵ��1��2��ʵ��2���¶ȸߣ��¶����ߣ�ƽ�������ȷ����ƶ����÷�ӦΪ���ȷ�Ӧ������ƽ��������Ӧ�����ƶ���ƽ�ⳣ������K2��K1������K2��K1=K3��
�ʴ�Ϊ��480��303��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죻K2��K1=K3����
���������⿼�����¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ���֪ʶ�㣬�ѶȲ���ע�⻯ѧƽ�ⳣ��ֻ���¶��йأ��������������أ�

��1��������һ������Ľྻȼ�ϣ���֪��
CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-802.3kJ?mol-1
H2O��1��=H2O��g����H=+44.0kJ?mol-1
��4.8g����������ȫȼ������Һ̬ˮ���ų�����Ϊ______��
��2�����ü�����ˮ��Ӧ�Ʊ���������ԭ�����ۣ������ƹ��ֵ��
�÷�ӦΪCH4��g��+H2O��g��
����800��ʱ����Ӧ�Ļ�ѧƽ�ⳣ��K=l.0��ijʱ�̲�ø��¶����ܱ������и����ʵ����ʵ���Ũ�����±���
| CH4 | H2O | CO | H2 |
| 3.0mol?L-1 | 8.5mol?L-1 | 2.0mol?L-1 | 2.0mol?L-1 |
A��v��������v���棩 B��v��������v���棩C��v������=v���棩D�����ж�
��Ϊ��̽���¶ȡ�ѹǿ��������ѧ��Ӧ���ʵ�Ӱ�죬��ɽͬѧ�������������Ա�ʵ�飨�¶�Ϊ360���480�桢ѹǿΪ101kPa��303kPa������ʵ���������±�����
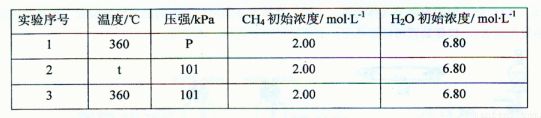
| ʵ����� | �¶�/�� | ѹǿ/kPa | CH4��ʼŨ��/mol?L-1 | H2O��ʼŨ��/mol?L-1 | K |
| 1 | 360 | P | 2.00 | 6.80 | K1 |
| 2 | t | 101 | 2.00 | 6.80 | K2 |
| 3 | 360 | 101 | 2.00 | 6.80 | K3 |
���ʵ��2��3��Ŀ����______��
ʵ��l��2��3�з�Ӧ�Ļ�ѧƽ�ⳣ���Ĵ�С��ϵ��______����K1��K2��K3��ʾ����
��3�����ô�����ͨ��������Ӧ�ɽ�ˮ�ֽ��Ƶ�����������һ����ӦΪ��MnFe2O4
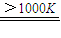 MnFe2O4-x+
MnFe2O4-x+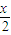 ���������������Ϊ______��
���������������Ϊ______���ڶ�����Ӧ�Ļ�ѧ����ʽΪ______���ɲ�д��Ӧ��������
 CO��g��+3H2��g����H=+206.1kJ?mol-1��
CO��g��+3H2��g����H=+206.1kJ?mol-1�� CO��g��+3H2��g��;��H=+206��lkJ��mol-l��
CO��g��+3H2��g��;��H=+206��lkJ��mol-l��