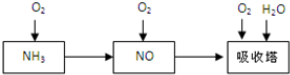
��Ŀ����
1������þ[Mg��ClO3��2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg��ClO3��2•6H2O���������£�
��֪����±����Ҫ�ɷ�ΪMgCl2•6H2O������MgSO4��FeCl2�����ʣ�
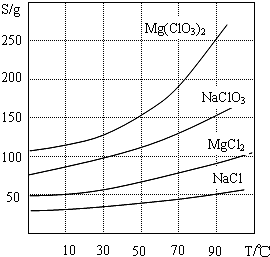
�����ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ��
��1����������Ҫ����Ҫ����������©�������������ձ���
��2������BaCl2��Ŀ���dz�ȥSO42-��ʹSO42-��������MgO�����������������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪMgCl2+2NaClO3=Mg��ClO3��2+2NaCl�����ٽ�һ����ȡMg��ClO3��2•6H2O��ʵ�鲽������Ϊ�����ȹ��ˣ�����Һ����ȴ�ᾧ
�������ᾧ���ڣ�ϴ�ӣ��������ȥ���ۣ��ܹ��ˡ�ϴ�ӣ�
��4����Ʒ��Mg��ClO3��2•6H2O�����IJⶨ������֪Mg��ClO3��2•6H2O��Ħ������Ϊ299g/mol��
����1��ȷ����3.50g��Ʒ���100mL��Һ��
����2��ȡ10.00mL����ƿ�У�����10.00mLϡ�����20.00mL 1.000mol•L-1��FeSO4��Һ���ȣ�
����3����ȴ�����£���0.100mol/L K2Cr2O7��Һ�ζ�ʣ���Fe2+���յ㣮
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7��Һ15.00mL��
��д������2�з�����Ӧ�����ӷ���ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
��д������3�з�����Ӧ�����ӷ���ʽ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
�۲�Ʒ��Mg��ClO3��2•6H2O����������Ϊ78.3%��
���� ±����Ҫ�ɷ�ΪMgCl2.6H2O������MgSO4��FeCl2�����ʣ�������������������������������Ϊ�����ӣ������Ȼ�����Һ�������ᱵ��������������þ��������Һ��pHΪ4����ʱ�������γ��˳�����������������NaClO3������Һ������ӦΪ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����ˣ��õ�Mg��ClO3��2��Һ�У��������ʵ��ܽ�ȴ�С����Һ��þ���ķ����������ᾧ�����ˡ���ȴ�ᾧ��ȡMg��ClO3��2•6H2O��
��1�����˲��õ�������������̨��©�����ձ����������ȣ��������ڲ��������У�©�����ձ�����������
��2������BaCl2��Һ��Ŀ���dz�����������ӣ����ݼ�����þ����Һ��pHΪ4�����Գ������������ش�
��3������NaClO3������Һ���Ȼ�þ�������Ʒ������ֽⷴӦ��������þ���Ȼ��Ƴ������������ʵ��ܽ�ȴ�С����Һ��þ���ķ����������ᾧ�����ˡ���ȴ�ᾧ��
��4������������Ӿ��������ԣ����Խ�������������Ϊ�����ۣ�
��Cr2O72-�ܽ�Fe2+������Fe3+�����ݵ��ӵ�ʧ�غ��д���ӷ���ʽ��
�۸��ݻ�ѧ��ӦClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+�����ݸ�������֮��Ĺ�ϵʽ�ɼ���ٷֺ�����
��� �⣺��1�����˵�ԭ���������ǰѲ�����Һ��Ĺ������ʸ�Һ����뿪����һ�ֻ�������ķ���������ʱ��Ҫ������������©������ֽ���̶�����������̨�������õIJ��������н���Һ���ձ��ȣ�������Ҫʹ�õIJ����������ձ�����������©����
�ʴ�Ϊ���ձ�����������©����
��2��±����Ҫ�ɷ�ΪMgCl2.6H2O������MgSO4��FeCl2�����ʣ������Ȼ�����Һ�������ᱵ���������Լ���BaCl2��Һ��Ŀ���dz�ȥSO42-����������þ��������Һ��pHΪ4����ʱ�������γ��˳�����������������������������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
�ʴ�Ϊ����ȥSO42-��ʹSO42-������BaSO4��Fe��OH��3��
��3������NaClO3������Һ������ӦΪ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����������ʵ��ܽ�ȴ�С����Һ���Mg��ClO3��2•6H2O����ķ����������ᾧ�����ˡ���ȴ�ᾧ��
�ʴ�Ϊ��MgCl2+2NaClO3=Mg��ClO3��2+2NaCl�������ȹ��ˣ�����Һ����ȴ�ᾧ��
��4������������Ӿ��������ԣ����Խ�������������Ϊ�����ۣ���ѧ����ʽΪ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
�ʴ�Ϊ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
��Cr2O72-�ܽ�Fe2+������Fe3+����Ӧ�����ӷ���ʽΪCr2O72-+6Fe2++14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+=6Fe3++2Cr3++7H2O��
�۸��ݻ�ѧ����ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+����0.100mol•L-1 K2Cr2O7��Һ�ζ����յ���̿��Եó�ʣ����������ӵ����ʵ���Ϊ��0.100mol•L-1��0.015L��6=0.009mol������������ӷ�Ӧ���������ӵ����ʵ���Ϊ��20��10-3L��1.000mol•L-1-0.009mol=0.011mol����������ӵ����ʵ���Ϊ��$\frac{1}{6}$��0.011mol����Ʒ��Mg��ClO3��2•6H2O��������������$\frac{1}{2}$��$\frac{1}{6}$��0.011��299g/mol����10��$\frac{1}{3.5}$��100%=78.3%��
�ʴ�Ϊ��78.3%��
���� ���⿼��Գ�������Ԫ�ؼ��仯������Ҫ���ʵ����գ��Լ������ӷ�Ӧʵ�ʵ���ʶ��ͬʱ����Ӧ�û���֪ʶ�����ѧ����������Լ���ͼ���Ĺ۲졢���������������ܽ�ȸ����Ӧ�ã����������ķ������������ӵij���ԭ����Լ�ѡ���Ƕ�ѧ���ۺ������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�| A�� | ��ҵ�ϳ�ʹ�ù����Ŀ�����Ŀ����Ϊ��ʹSO2��ת���ʴ�100% | |
| B�� | ��ҵ��ѡ��V2O5���÷�Ӧ�Ĵ�����Ŀ����Ϊ�˼ӿ컯ѧ��Ӧ���� | |
| C�� | ��ҵ�ϳ�ѡ�ó�ѹ��ԭ��������ѹǿ���ܸı�÷�Ӧ�Ļ�ѧ��Ӧ���� | |
| D�� | �����¶ȿ��Լӿ췴Ӧ���ʣ������ڹ�ҵ�����и÷�Ӧ�¶�Խ��Խ�� |
| A�� | CuCl2 | B�� | FeCl2 | C�� | FeCl3 | D�� | AlCl3 |
| A�� | ��m+4.8��g | B�� | ��m+5.1��g | C�� | ��m+10.2��g | D�� | ��m+19.2��g |
 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.01 | 0.008 | 0.007 | 0.007 | 0.007 |
��2����֪�÷�Ӧ��Ӧ������������������������������÷�Ӧ�Ƿ��ȣ�����ȡ������ȡ�����Ӧ��
��3����O2��ʾ��0-2s�ڸ÷�Ӧ��ƽ������v��O2��=1.5��10-3mol•L-1•s-1��
��4����ͼ�б�ʾc��NO2����ʱ��ı仯������b��
| A�� | ��״���£�5.6LO2��5.6LNO��Ϻ�ķ�������Ϊ0.5NA | |
| B�� | ��״���£�22.4���������к���6NA��C-C�Ҽ���6NAC-H�Ҽ� | |
| C�� | 7.8�˹��������к�0.2NA�����Ӻ�0.1NA������ | |
| D�� | ��500ml0.1mol•L-1NaAlO2��Һ��AlO${\;}_{2}^{-}$��Ϊ5��10-2NA |

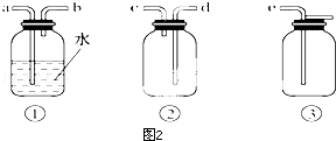

 ʵ����������ͼ��ʾװ����ȡ��������ˮCuCl2���Իش��������⣺
ʵ����������ͼ��ʾװ����ȡ��������ˮCuCl2���Իش��������⣺