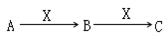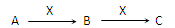��Ŀ����
��8�֣�����3�ֻ�����A��B��C����������Ԫ��R����ת����ϵ����ͼ��ʾ��
��1����A�ɵ�������2��Ԫ����ɡ������£�0.1mol/L X��Һ��pH��13����R�������е�λ����______________��X�������ӵĵ���ʽ��_________��Bת��ΪC�����ӷ���ʽ��___________��
��2����������A��B��C��X��Ϊ��̬���ʣ�1mol A�к��й��ۼ�����ĿԼΪ1.806��1024��XΪ���ʣ�A��X��Ӧ����B�Ļ�ѧ����ʽ��_____________����һ�������£�A����C��Ӧ����C�Դ�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽ��________________��
��8�֣�
��1����1�֣��������ڢ�A�� ��1�֣�![]() ��2�֣�Al(OH)3��OH����AlO2����2H2O
��2�֣�Al(OH)3��OH����AlO2����2H2O
��2����2�֣�4NH3��5O24NO��6H2O ��2�֣�8NH3��6NO2
7N2��12H2O
����:��

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д�
�����Ŀ
 ����3�ֻ�����A��B��C����������Ԫ��R����ת����ϵ��ͼ��ʾ��
����3�ֻ�����A��B��C����������Ԫ��R����ת����ϵ��ͼ��ʾ��
 A��B��C����������Ԫ��R����ת����ϵ����ͼ��ʾ��
A��B��C����������Ԫ��R����ת����ϵ����ͼ��ʾ��