��Ŀ����
ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L H2SO4��Һ�����к��ȵIJⶨ��
��.����0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ����������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(�����) ��
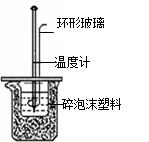
��.�ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ
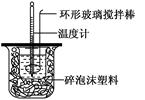
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ��mol��1)��_______________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL�������ʵ�飬ʵ���������±���
������д�±��еĿհף�
�ڸ�������ʵ�����ݼ�������к���Ϊ53.5 kJ/mol�������к��ȵ�����ֵ57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a��ʵ��װ�ñ��¡�����Ч����
b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��.����0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ����������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(�����) ��
| ���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� | 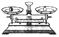 |  |  |  |  | 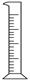 |
| ��� | a | b | c | d | e | f |
��.�ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ

��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ��mol��1)��_______________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL�������ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ƽ���¶Ȳ� (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڸ�������ʵ�����ݼ�������к���Ϊ53.5 kJ/mol�������к��ȵ�����ֵ57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a��ʵ��װ�ñ��¡�����Ч����
b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��1��5.0 ��2��abe
��1��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l) ��H="-57.3KJ/mol" ��2�� �� 4.0 ��a c d
��1��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l) ��H="-57.3KJ/mol" ��2�� �� 4.0 ��a c d
�����������1������ƿ��245 mL NaOH��Һ�ӽ��Ĺ����250 mL�ġ���Һ�о�һ�ԣ�������Ũ����ͬ��n(NaOH)="V��C=0.25L��0.50" mol/L="0.125" mol.m(NaOH)="n��M=0.125" mol��40g/mol=5.0g.��2����������ҩƷҪ����ƽ��ҩ�ס��������������и�ʴ�����Բ���ֱ������ƽ��������Ҫ�����ձ��г�������˳���NaOH��������Ҫ������������a b e��1���к�����������Ӧ����1Ħ����ˮʱ�ų����ȡ�ǿ����ǿ����кͷ�Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽΪ��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l) ��H="-57.3KJ/mol" ��2���ٷ��������������ݿ��Կ������ڶ����������̫��Ҫȥ������������������ݵ��¶Ȳ��ƽ��ֵ����ƽ���¶Ȳ{��30.1-26.1��+��29.8-25.9��+��30.4-26.3��}��3=4.0�����к��ȵ�ʵ��ֵΪ53.5 kJ/mol���к��ȵ�����ֵ57.3 kJ/molС�����ܵ�ԭ����a��ʵ��װ�ñ��¡�����Ч���c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȡ�����b������ȡNaOH��Һ�����ʱ���Ӷ��������������Ƶ����ƫС��������ˮ�����ʵ���ƫС�к��ȵ���ֵ��ƫ���������Υ�����ʲⶨ���к���ƫС��ԭ����a c d��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
�����Ŀ