��Ŀ����
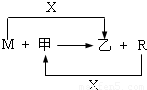
M��R���������г����Ľ������ʣ�����R���������Ľ������ס����ǻ�������м��Ǻ�ɫ���壬����R��X��ȼ�յõ���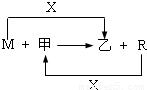
��1��M����ڸ����·�Ӧ�Ļ�ѧ����ʽ�� ��
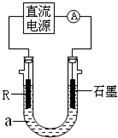
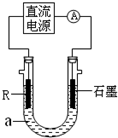
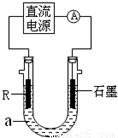
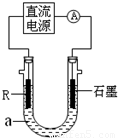
��2����ⷨ��R�ͼ����������װ����ͼ��a��4mol?L-1NaCl��1mol?L-1NaOH�Ļ����Һ��
������aʱ��Ҫ��ȥ����ˮ���ܽ��O2�������� �ķ�����
��ʯī�缫Ӧ���Դ�� ��������������������ӣ�ͨ���R�缫������������ ��R���ĵ缫��Ӧʽ�� ��
��ֹͣʵ��һ��ʱ�����R���ϲ��к��ɫ���ʲ�������Ӧ�Ļ�ѧ����ʽ�� ��
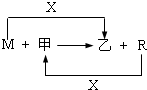
��3����R��ij�������ĩ��M��ĩ��Ϻ�ֳ����ȷݣ�һ���ڸ�����ǡ����ȫ��Ӧ�������������ᷴӦ����һ��ֱ�ӷ����������ռ���Һ�г�ַ�Ӧ��ǰ��������������ɵ�������������a��b����R��������Ļ�ѧʽ�� ��
���𰸡���������1���������ȷ�Ӧ��ԭ������д����ʽ��
��2���ټ��������Һ�ܽ���Һ�е��������ʳ�ȥ��
�ڽ������������ǣ��缫����ʧȥ���ӱ�Ϊ�������ӣ������������ӷ�Ӧ��������������������
���������������������ױ���������Ϊ����������
��3���������������Լ��������ƾ��ܷ�Ӧ�����Ҿ���������
����⣺��1�����������ͼʾ���ݣ�R���������Ľ�������֪R�ǽ����������Ǻ�ɫ���壬����֪�����������������ܽ������������е����û������ķ�Ӧԭ�������ȷ�Ӧԭ����
�ʴ�Ϊ��8Al+3Fe3O4 4Al2O3+9Fe��
4Al2O3+9Fe��
��2������������������ȡ������ܿ������е����������������Һ�ܽ���Һ�е��������ʳ�ȥ���ʴ�Ϊ����У�
�ڵ�ⷨ��ȡ����������ʱ���������������������ڸõ缫�ϣ�������ʧ���ӣ�����ʯī��������ʯī�缫Ӧ���Դ�ĸ����������������������ɵ��������ӻ����������Ӧ����������������ɫ�������ʴ�Ϊ���������ɰ�ɫ������Fe-2e-+2OH-=Fe��OH��2����
���������������������ױ���������Ϊ������������Ӧ�Ļ�ѧ����ʽ�ǣ�4Fe��OH��2 +O2+2H2O�T4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2 +O2+2H2O�T4Fe��OH��3��
��3�����ɵ�������������a��b��Ҳ�������ʵ���֮��Ϊa��b����һ���ڸ�����ǡ����ȫ��Ӧ�������������ᷴӦ����Fe+2HCl=FeCl2+H2������������������ʵ���Ϊa�������Ľ����������ʵ���Ϊa����һ��ֱ�ӷ����������ռ���Һ�г�ַ�Ӧ����2Al+6H2O+2NaOH=2Na[Al��OH��4]+3H2�����������������ʵ���Ϊb�������Ľ���������Ϊ ��������������ͽ������ķ�Ӧ�У��跴ӦΪ��FexOy+Al��Al2O3+Fe������ԭ���غ㣬�����������ʵ���Ϊa����x=a�������������ʵ���Ϊ
��������������ͽ������ķ�Ӧ�У��跴ӦΪ��FexOy+Al��Al2O3+Fe������ԭ���غ㣬�����������ʵ���Ϊa����x=a�������������ʵ���Ϊ ʱ�����������е���ԭ�����ʵ���Ϊb����y=b��
ʱ�����������е���ԭ�����ʵ���Ϊb����y=b��
�ʴ�Ϊ��FeaOb��
������������Ŀ�Ƚϸ��ӣ���������ݱȽ϶࣬�Ѷ�Ҳ�ܴ���ѧ�������ͽ������ĸ���������
��2���ټ��������Һ�ܽ���Һ�е��������ʳ�ȥ��
�ڽ������������ǣ��缫����ʧȥ���ӱ�Ϊ�������ӣ������������ӷ�Ӧ��������������������
���������������������ױ���������Ϊ����������
��3���������������Լ��������ƾ��ܷ�Ӧ�����Ҿ���������
����⣺��1�����������ͼʾ���ݣ�R���������Ľ�������֪R�ǽ����������Ǻ�ɫ���壬����֪�����������������ܽ������������е����û������ķ�Ӧԭ�������ȷ�Ӧԭ����
�ʴ�Ϊ��8Al+3Fe3O4
 4Al2O3+9Fe��
4Al2O3+9Fe����2������������������ȡ������ܿ������е����������������Һ�ܽ���Һ�е��������ʳ�ȥ���ʴ�Ϊ����У�
�ڵ�ⷨ��ȡ����������ʱ���������������������ڸõ缫�ϣ�������ʧ���ӣ�����ʯī��������ʯī�缫Ӧ���Դ�ĸ����������������������ɵ��������ӻ����������Ӧ����������������ɫ�������ʴ�Ϊ���������ɰ�ɫ������Fe-2e-+2OH-=Fe��OH��2����
���������������������ױ���������Ϊ������������Ӧ�Ļ�ѧ����ʽ�ǣ�4Fe��OH��2 +O2+2H2O�T4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2 +O2+2H2O�T4Fe��OH��3��
��3�����ɵ�������������a��b��Ҳ�������ʵ���֮��Ϊa��b����һ���ڸ�����ǡ����ȫ��Ӧ�������������ᷴӦ����Fe+2HCl=FeCl2+H2������������������ʵ���Ϊa�������Ľ����������ʵ���Ϊa����һ��ֱ�ӷ����������ռ���Һ�г�ַ�Ӧ����2Al+6H2O+2NaOH=2Na[Al��OH��4]+3H2�����������������ʵ���Ϊb�������Ľ���������Ϊ
 ��������������ͽ������ķ�Ӧ�У��跴ӦΪ��FexOy+Al��Al2O3+Fe������ԭ���غ㣬�����������ʵ���Ϊa����x=a�������������ʵ���Ϊ
��������������ͽ������ķ�Ӧ�У��跴ӦΪ��FexOy+Al��Al2O3+Fe������ԭ���غ㣬�����������ʵ���Ϊa����x=a�������������ʵ���Ϊ ʱ�����������е���ԭ�����ʵ���Ϊb����y=b��
ʱ�����������е���ԭ�����ʵ���Ϊb����y=b���ʴ�Ϊ��FeaOb��
������������Ŀ�Ƚϸ��ӣ���������ݱȽ϶࣬�Ѷ�Ҳ�ܴ���ѧ�������ͽ������ĸ���������

��ϰ��ϵ�д�
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ