��Ŀ����
��10�֣������������С��
��1�������г��˼������ʣ���ѡ��������ʵ�������ڿո��ϡ�
ͬλ�� ��ͬϵ�� ��ͬ���칹�� ��
�� ���ʯ��ʯī ��CH3CH2CH2CH3��(CH3)2CHCH3
��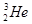 ��
��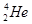 ��CH3CH3��CH3CH2CH2CH3
��CH3CH3��CH3CH2CH2CH3 �� ��CH2��CHCH3��CH2��CH2��
�� ��CH2��CHCH3��CH2��CH2��
��D��T������ ��������������������������������
��2���й��ľ��Ļ�ԨԴ�������Ŵ����DZ㶮��������ʳ����ʵ���������ƣ���д����������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��___________________��
���Ҵ��������Ļ�ѧ����ʽ��______________________________��
����10�֣�ÿ��2��)��1�� �ۢ� �ܢ� �ڢ�
��2����C6H12O6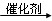 2CH3CH2OH��2CO2��
2CH3CH2OH��2CO2��
��2C2H5OH+O2 2CH3CHO+2H2O
2CH3CHO+2H2O
����

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ
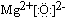
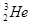 ��
��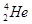 ��CH3CH3
��CH3CH2CH2CH3
��CH3CH3
��CH3CH2CH2CH3 ��
��CH2��CHCH3��CH2��CH2��
��
��CH2��CHCH3��CH2��CH2��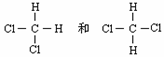 �� ��CH2��CHCH3��CH2��CH2��
�� ��CH2��CHCH3��CH2��CH2��