��Ŀ����
ij��ѧС��ͬѧΪ����֤�ճ��������û��ͷ�еĻ�ѧ�ɷ֣���KClO3��MnO2��S�ȣ������������ʵ�����̣���ͼ-1����
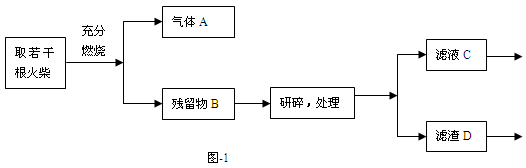
�Իش��������⣺
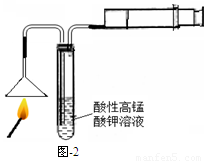
��1��ȼ�ŵĻ��ͼ-2����ʵ�飬���Թ����ܹ۲쵽 ���������֤�����ͷ�к�����Ԫ�ء�ͼ����Ͳ�������� ��
��2��Ϊ��֤�����ͷ�к�����Ԫ�أ�������ʵ�鲽���� ��
��3����ͬѧ���������ͷ��KClO3����һʵ�鷽����
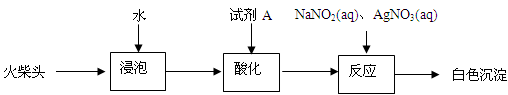
�Լ�AΪ ������NaNO2��Ŀ���� ��
��4�����ʵ�飺������֤����D�к���MnO2��һ��ʵ�鷽������д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��1��KMnO4��Һ��ɫ����ɫ��dz��������ȷ������2�֣���
����˼��ȷ���ɣ���1�֣�
��2��ȡ������ҺC�����Թ��У��Ⱥ�����HNO3��AgNO3��Һ�����۲쵽��ɫ����������������֤�����ͷ�к�����Ԫ�ء���1�֡�3��
��3�������HNO3��1�֣�����ԭKClO3��1�֣�
��4��ȡ��������D����װ������H2O2���Թ��У��д������ݷų�˵������MnO2��2�֣���
2H2O2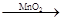 2H2O+O2����2�֣���������ȷ�𰸡�
2H2O+O2����2�֣���������ȷ�𰸡�
��������
�����������1��������Ԫ��ȼ���������˶������������ʹKMnO4��Һ��ɫ����ɫ��dz������Ͳ����ʹȼ�ղ���������˳�������Թܡ�
��2����Ԫ��ȼ������Ҫ�������Ȼ������壬ֻҪȡ������ҺC�����Թ��У��Ⱥ�����HNO3��AgNO3��Һ�����۲쵽��ɫ����������������֤�����ͷ�к�����Ԫ�ء��൱���������ӵļ��顣
��3��KClO3��ǿ�������ԣ������Ի����£���������HNO3��Ҫ���ữ�������������н�ǿ�Ļ�ԭ�ԣ����Ի�ԭKClO3Ϊ�����ӡ�
��4����֤�Ƿ���MnO2��������ʵ������ȡ�����ķ�����Ҳ������Ϊ����ȡ��������D����װ������H2O2���Թ��У��д������ݷų�˵������MnO2����ط�Ӧ 2H2O2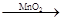 2H2O+O2��
2H2O+O2��
���㣺������ʵ��̽����˼·��Ԫ�ػ������еĻ���֪ʶ��

����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ����������������������������������������������������
��2������������ʵ���������������������������������������
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ��������������������������������
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ�
��ԭ�������������������������
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(���ػ���)��
c������������ ������ ��
����15�֣�
��֪����25ʱH2OH++OH-��KW=10-14�� CH3COOH
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH��
�ı�ֵ�� ���������С�����䡱��
��2��������ˮ������ӷ���ʽΪ�������������������������¶�ʱ��C(OH��)����������
���������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д�����
�ı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵ
Ϊ������������������ ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������pH��3��HA��ҺV1mL��pH��11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ���������
�������� �����ɳ�����ԭ�������� ��
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ����
����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ�������������������������������������������������� ��
��2������������ʵ������������������������������������� ��
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ������������������������������ ��
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��

��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
|
����NaOH��Һ���/mL |
26.02 |
25.35 |
25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ�
��ԭ����������������������� ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(���ػ���)��
c������������ ������ ��
����15�֣�
��֪����25ʱH2O H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH
 H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH��
�ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ���������������� ���������¶�ʱ��C(OH��)����������
���������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д�����
�ı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵ
Ϊ������������������ ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������pH��3��HA��ҺV1mL��pH��11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ���������
�������� �����ɳ�����ԭ�������� ��
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ����



