��Ŀ����
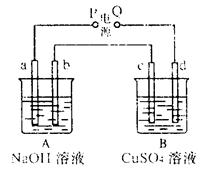
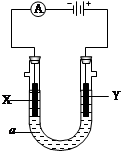
(11��)���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
(3)��ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��
������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ.
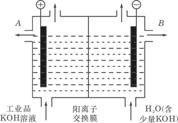
�ٸõ��۵�������Ӧʽ��____________________________.
��ͨ�翪ʼ������������ҺpH�����������ԭ��
�۳�ȥ���ʺ������������Һ����Һ����_________________(��д��A����B��)����

��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
(3)��ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��
������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ.

�ٸõ��۵�������Ӧʽ��____________________________.
��ͨ�翪ʼ������������ҺpH�����������ԭ��
�۳�ȥ���ʺ������������Һ����Һ����_________________(��д��A����B��)����
��11�֣��Ţ�2H+ + 2e - = H2�� �ų����壬��Һ��졣
��2Cl - - 2e - = Cl2 �� ����ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ��
�Ƣٴ�ͭ Cu2+ + 2e - = Cu �ڴ�ͭCu - 2e - = Cu2+
��3����4OH����4e����O2����2H2O ����2H+ + 2e - = H2��ʹH+Ũ�ȼ�С ��B
��2Cl - - 2e - = Cl2 �� ����ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ��
�Ƣٴ�ͭ Cu2+ + 2e - = Cu �ڴ�ͭCu - 2e - = Cu2+
��3����4OH����4e����O2����2H2O ����2H+ + 2e - = H2��ʹH+Ũ�ȼ�С ��B
�����������1����X�͵�Դ�ĸ�������������������Һ�е������ӷŵ������������缫��Ӧʽ��2H+ + 2e - = H2�����������������ӵIJ��Ϸŵ磬������Χˮ�ĵ���ƽ�ⱻ�ƻ�������������Χ��Һ�Լ��ԣ���Һ��ɺ�ɫ��
��Y�缫�͵�Դ����������������������Һ�е������ӷŵ������������缫��Ӧʽ��2Cl - - 2e - = Cl2����������������ǿ�����ԣ��ܰѵ⻯���������ɵ��ʵ⣬�ݴ˿��Լ��飬������ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ��
��2����ͭ����ʱ����ͭ����������ͭ������������ͭ���������Һ������X�Ǵ�ͭ���缫��Ӧʽ��Cu2+ + 2e - = Cu��Y�缫�Ǵ�ͭ���缫��Ӧʽ��Cu - 2e - = Cu2+��
��3������������Һ�е�OH�����ӷŵ������������缫��Ӧʽ��4OH����4e����O2����2H2O��
�����������ӷŵ�ʧȥ�������缫��ӦʽΪ2H+ + 2e - = H2�����������������ӵIJ��Ϸŵ磬������Χˮ�ĵ���ƽ�ⱻ�ƻ���ʹH+Ũ�ȼ�С��OH��Ũ��������Һ�Լ��ԡ�
�����������ӽ���Ĥֻ������������ͨ������OH�����������ɡ�����װ��ͼ��֪��B�缫�����������Գ�ȥ���ʺ������������Һ����Һ����B������
�������������ؼ�����ȷ�жϳ��缫�����ӵķŵ�˳�����жϵ�����ʱ�������ж������缫���ϡ�����ǻ��Ե缫����缫����ʧȥ���ӡ�����Ƕ��Ե缫������Һ�е�������ʧȥ���ӡ�����������Һ�е������ӵõ����ӣ�������Ҫ������ס�������ӵķŵ�˳��

��ϰ��ϵ�д�
�����Ŀ