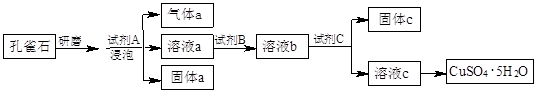��Ŀ����
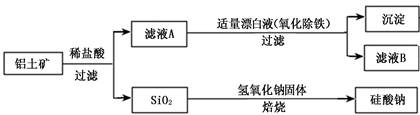
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
������ʯ�к������IJⶨ��
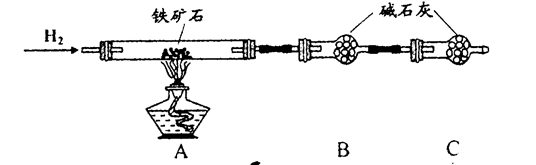
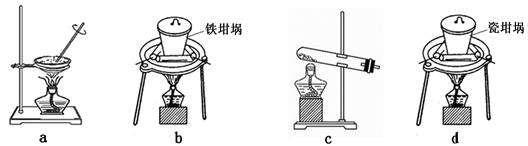
�� ����ͼ��װ���������װ�õ������ԣ�
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��Ϊ��ʯ��(���ͼʾ���г�����ʡ��)
�� ����˵����ܿڴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ �������� ��������������
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ�������� ��
������ʯ�к������IJⶨ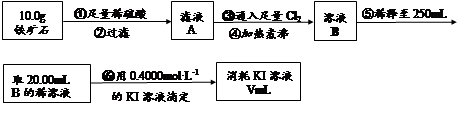
��3����������������������������������������������������������� ��
��4����������õ��IJ����������ձ�������������ͷ�ιܡ������� ���� ��
��5�������йز���IJ�����˵����ȷ���������������������� �������� ��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
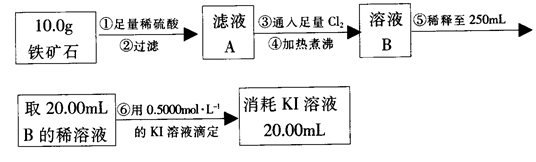
��6�����ζ�����������0.4000mol��L?1KI��Һ25.00ml��������ʯ�����İٷֺ���Ϊ�� ����
��7���ɢ���������������ʯ������������Ļ�ѧʽΪ����������
��15�֣���1����ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�������2�֣�
��2��24%��2�֣� ��3��������Һ���ܽ�Ĺ�����Cl2 ��2�֣�
��4��250mL����ƿ��2�֣���δ��250mL�������֣�
��5��df ��2�֣� ��6��70% ��2�֣� ��. ��7��Fe5O6 ��3�֣�
���������������1����ʵ���У���������������Ӧ���ɽ�������ˮ�����ݹ��������ı仯���������ĺ�����B���ĸ�������������ղ�����ˮ����������Cװ��Ҫ��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�����
��2����ķ�Ӧ��װ��B����1.35g������B���ĸ�������������ղ�����ˮ�������������ӵ���������ˮ����������������ˮ����Ԫ�����������������������Ԫ�ص�������1.35g�� ��1.2g��������Ԫ�ص�����������
��1.2g��������Ԫ�ص�����������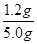 ��100%��24%��
��100%��24%��
��3��������ʯ�м������ᣬ��Ӧ���������������Һ����������������ǰ���Һ�е����������������������ӡ����������ǹ����ģ�����Ҳ�����������ӣ�������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2��
��4������ƿ��һ�ֶ�������������ϡ�͵�250mL������õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ.
��5��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b���ζ������У����������Ժ͵����ӷ�����Ӧ�����������Ӻ͵ⵥ�ʣ��ⵥ������������Һ��ʾ��ɫ�������������ӵ���ɫ������������ã���b����
c���ζ���������ˮϴ�Ӻ�����ñ�Һ��ϴ����c����
d����ƿ����Ҫ�ô���Һ��ϴ����d��ȷ��
e���ζ������У��۾�ע����ƿ����ɫ�ı仯����e����
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������f��ȷ����ѡdf��
��6�����ݷ���ʽ2Fe3����2I����I2��2Fe2����֪��c(Fe3+)��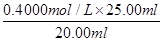 ��0.5mol/L��������Ԫ�صİٷֺ�����
��0.5mol/L��������Ԫ�صİٷֺ����� ��100%=70%��
��100%=70%��
��7���ɢ���������������ʯ��������ԭ�ӵĸ���֮���� :
: ��5:6����˻�ѧʽ��Fe5O6��
��5:6����˻�ѧʽ��Fe5O6��
���㣺���黯ѧʵ����������������仯��������ʡ�ת���Լ���ѧʽ�ⶨ���йؼ�����жϵ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ijͬѧ��һ����������Cl2ǡ����ȫ��Ӧ�õ�һ��������R��Ȼ��ͨ��ʵ��ȷ����ɷ֡�̽���������£�
��1��������裺��������м��貹��������
����A���ù���������FeCl3��
����B��__________________________________________________________��
����C��__________________________________________________________��
��2�����ʵ�鷽����
ȡ������������R���ձ��У�������ˮ�ܽ⣬Ȼ��ȡ����R��Һ�ֱ����ʵ�飬ʵ��������������±������ڱ�������дʵ������
| ʵ�鷽�� | ʵ������ | ���� |
| ����R��Һ�� ��KSCN��Һ | | ������������FeCl3 |
| ����R��Һ�еμ� ����KMnO4��Һ | | ���������в���FeCl2 |
�ɴ˵ó����ۣ�����________����������ĸ����
��3��д����R��Һ�еμ�����KSCN��Һ�����ӷ���ʽ�� ��25��Cʱ����ø÷�Ӧ��ƽ��ʱ��ƽ�ⳣ��ΪK1�������¶Ȳ��䣬�����μ�����KSCN��Һ������ƽ��ʱ�����ƽ�ⳣ��ΪK2����K1 K2�������������������������Һ����ɫ ������������dz�����䡱����
��4��R��Һ����ӡˢ��·ͭ��ĸ�ʴ����д���÷�Ӧ�����ӷ���ʽ��___________________________��
������������������ͭ���Ǻ�ɫ��ĩ�����������ϡ�ijУ��ѧʵ��С��ͨ��ʵ��̽��ij��ɫ��ĩ��Fe2O3��Cu2O�������̽���������£�
�������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4��
���̽��ʵ�飺ȡ������ĩ��������ϡ�����У���������Һ�еμ�KSCN�Լ���
��1����ֻ��Fe2O3����ʵ��������_____________��
��2���������ĩ��ȫ�ܽ�������ڣ��μ�KSCN�Լ�ʱ��Һ�����ɫ����˹����е����ӷ�ӦΪ��
____________________________��
��3����ʵ�������ȷ����ɫ��ĩΪCu2O��Fe2O3�Ļ���ʵ��С�����ⶨCu2O��������������֪Cu2O�ڿ����м�������CuO��
�ⶨ���̣�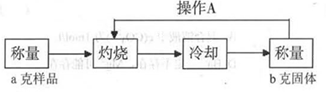
ʵ���в���A������Ϊ_____________��
���չ����У����������У��ƾ��ơ���������_____________�ȣ��г��������⣩��
��4��д���������Cu2O�����������ı���ʽ_____________��
ʵ��С�������ú�ɫ��ĩ��ȡ�ϴ����ĵ�����CuSO4?5H2O�������������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2+��Fe2+��Fe3+�ֱ����ɳ�����pH���£�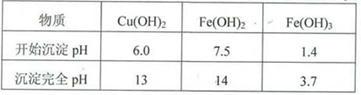
ʵ�����������Լ��ɹ�ѡ��
| A����ˮ | B��H2O2 | C��NaOH | D��Cu2(OH)2CO3 |

��5�����Լ���ű�ʾ���Լ�lΪ_____________���Լ�2Ϊ_____________��
��6��Ϊʲô�ȡ�����������pH����pH���Ʒ�ΧΪ���٣�__________________________________
��ȥ�����л��е��������Ȼ������壬���ѡ��
| A��ˮ | B������ʳ��ˮ |
| C��������Һ | D�������ռ���Һ |
һ���������������������Ӧ����0.25 g�����ĵ�314 mL����(��״��)���������PCl3��PCl5�����ʵ���֮�Ƚӽ���(�� ��)
| A��3��1 | B��5��3 | C��2��3 | D��1��2 |