��Ŀ����
6��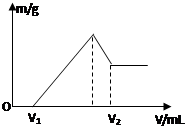 ��0.1molMg��Al���������100mL4mol/L�������У�Ȼ���ٵμ�1mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У���������m��NaOH��Һ�����V�ı仯��ͼ��ʾ��
��0.1molMg��Al���������100mL4mol/L�������У�Ȼ���ٵμ�1mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У���������m��NaOH��Һ�����V�ı仯��ͼ��ʾ����1����V1=140mLʱ���������n��Mg��=0.04mol��V2=460mL
��2�������NaOH��Һ400mL ʱ����Һ�е�Mg2+��Al3+�պó�����ȫ��
��3�����������Mg�����ʵ�������Ϊa���������NaOH��ҺΪ450mLʱ�����ó�������Al��OH��3����a��ȡֵ��Χ��0.5��a��1��
���� ��1����ͼ��֪���ӿ�ʼ������NaOH��ҺV1mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H++OH-=H2O��
�����μ�NaOH��Һ��������������ҺΪAʱ�����������ʱΪMg��OH��2��Al��OH��3����ʱ�����ķ�ӦΪ��Mg2++2OH-=Mg��OH��2��Al3++3OH-=Al��OH��3����ҺΪ�Ȼ�����Һ���ټ����μ�NaOH��Һ�������������������Ʒ�Ӧ����ƫ��������ˮ��������ӦAl��OH��3+OH-=AlO2-+2H2O��
��ͼ��֪��0��V1����H++OH-=H2O��V1��A����Mg2+��Al3+ת��Ϊ�����ķ�Ӧ��A��B�η���Al��OH��3+OH-=AlO2-+2H2O����Ϸ�Ӧ��ԭ���غ������
��2����þ���ӡ�������ǡ����ȫ����ʱ������Ϊ�Ȼ��ƣ���������غ㶨�ɼ������Ҫ����������Һ�����
��3�����������Ϊ0.1mol������þ�����ʵ�������Ϊa����400mL1mol•L-1�����ܽ���ټ���450mL 1mol•L-1����������Һ��������Һ��Al��OH��3������֤�����ɵ���������ȫ�����������Ʒ�Ӧ������������֪��v1=400ml���ټ���50mL 1mol•L-1����������Һ������Al��OH��3������ȫ����������������ƫ�����ƣ�����Al��OH��3������ȫ����������������ƫ�����ƣ���ã�Al��OH��3���ȡֵ0.05mol����0��n��Al����0.05mol���ٽ��n��Mg��+n��Al��=0.1mol�ж�a�ķ�Χ��
��� �⣺��1����ͼ��֪���ӿ�ʼ������NaOH��ҺV1mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��H++OH-=H2O��
�����μ�NaOH��Һ��������������ҺΪAʱ�����������ʱΪMg��OH��2��Al��OH��3����ʱ�����ķ�ӦΪ��Mg2++2OH-=Mg��OH��2��Al3++3OH-=Al��OH��3����ҺΪ�Ȼ�����Һ���ټ����μ�NaOH��Һ�������������������Ʒ�Ӧ����ƫ��������ˮ��������ӦAl��OH��3+OH-=AlO2-+2H2O��
��V1=140mL��˵��������ʣ�࣬ʣ����������ʵ���=1mol/L��0.14L=0.14mol����ͽ�����Ӧ����������ʵ���=4mol/L��0.1L-0.14mol=0.26mol��
��þ�����ʵ�����x���������ʵ�����y��x+y=0.1��2x=3y=0.26����ã�x=0.04mol��y=0.06mol��
V2ʱ��Һ�е�������ƫ�����ƺ��Ȼ��ƣ�����ԭ���غ�֪��n��NaOH��=n��Al��+n��HCl��=0.06mol+0.4mol=0.46mol�������������Ƶ����=$\frac{0.46mol}{1mol/L}$=460mL��
�ʴ�Ϊ��0.04��460��
��2����Һ�е�Mg2+��Al3+�պó�����ȫʱ����Һ������ΪNaCl����n��NaOH��=n��HCl��=4mol/L��0.1L=0.4mol����Ҫ����������Һ�����Ϊ��$\frac{0.4mol}{1mol/L}$=0.4L=400mL��
�ʴ�Ϊ��400��
��3�����������Ϊ0.1mol������þ�����ʵ�������Ϊa����400mL1mol•L-1�����ܽ���ټ���450mL 1mol•L-1����������Һ��������Һ��Al��OH��3������֤�����ɵ���������ȫ�����������Ʒ�Ӧ������������֪��v1=400mL���ټ���50mL 1mol•L-1����������Һ������Al��OH��3������ȫ����������������ƫ�����ƣ�Al��OH��3+NaOH=NaAlO2+2H2O��Al��OH��3���ȡֵ0.05mol����0��n��Al����0.05mol��n��Mg��+n��Al��=0.1mol����0.5��n��Mg����1��
�ʴ�Ϊ��0.5��a��1��
���� ������ͼ����ʽ����������㣬��Ŀ�Ѷ��еȣ���ȷÿһ��ͼ�����Ļ�ѧ��Ӧ��֪���յ�����ĺ��弰��Һ�����ʵijɷ֣���Ϸ���ʽ���й�������з�����ע���غ�˼������ã�����ʹ�����

| A�� | ������ͨ��ˮ | B�� | ������ͨ�����ʯ��ˮ | ||
| C�� | ������ͨ����ˮ | D�� | ������ͨ���������й��� |
| A�� | ���ȵĴ�����ϴ���� | B�� | ����������NaCl��Һ�� | ||
| C�� | �����ľ�ˮ���� | D�� | ��ĭ�������ʹ��ԭ�� |
| A�� | CH3CH��Br��OH | B�� | CH3CH��CH2OH��CHO | ||
| C�� | CH3CH2CH��CH3��CH2CH3 | D�� | HOOCCH��Br��COOCH3 |
| A�� | ����ЧӦ--CO2 | B�� | �⻯ѧ��Ⱦ--NO2 | C�� | ����--SO2 | D�� | �������ƻ�--CO |
| A�� | ԭ�Ӱ뾶��D��E��C��B��A | |
| B�� | ���ȶ��ԣ�EA4��A2C | |
| C�� | ����D���ú�ˮ��ԭ�ϻ�� | |
| D�� | ������DC�뻯����EC2�л�ѧ��������ͬ |
| A�� | ��������ˮ���ݵ�������������Ϊ������ѧ��ʴ | |
| B�� | ����Ƥ������п�������л���ʱ��������ֹ������ʴ | |
| C�� | �����ĸɵ�ز������ⶪ���������������� | |
| D�� | ���ܽ���������ˮ����ͭ��ˮ��ͷ���� |
| A�� | CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | |
| B�� | 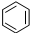 +HNO3$��_{��}^{Ũ����}$ +HNO3$��_{��}^{Ũ����}$ +H2O +H2O | |
| C�� | CH3CH2OH+CH3COOH $?_{��}^{Ũ����}$CH3COOCH2CH3+H2O | |
| D�� | CH2=CH2+HBr��CH3CH2Br |
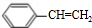 ��A�����д���ͬһƽ���ԭ�������16����
��A�����д���ͬһƽ���ԭ�������16����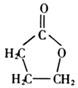 ��
��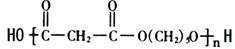 ��
��