��Ŀ����
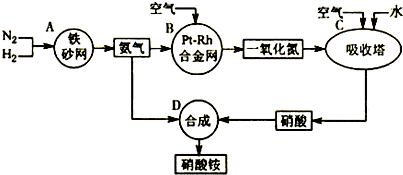
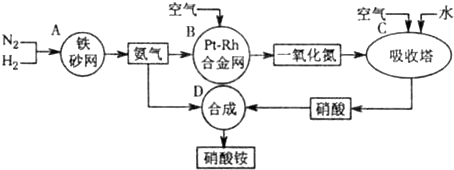
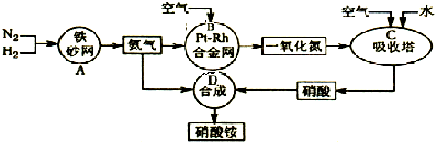
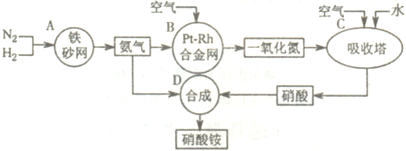
��ҵ��������淋�����ͼ���£�
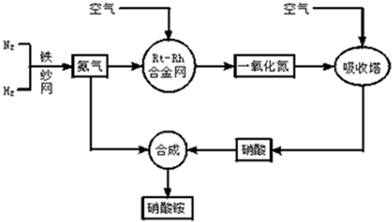
��ش��������⣺
��1��д��������ڹ�ũҵ�����е���Ҫ����______����дһ����
��2����֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1����ش�
����500�桢200atm��������������һ�ܱ������г���1molN2��3molH2����ַ�Ӧ�ų�������______���������������=����92.4kJ���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=______��
��Ϊ��Ч���������ת���ʣ��˲�ȡ�Ĵ�ʩ��______
A�������¶� B�����ʺϴ������Ե��ʵ����� C������ѹǿ
D������ѹǿ E��ѭ�����úͲ��ϲ��䵪�� F����ʱ�Ƴ���
��3��һ���¶���������̶����ܱ������У���1mol N2��3mol H2��ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ѹǿ�Ƿ�Ӧǰ��0.85������ʱN2��ת����Ϊ______��

��ش��������⣺
��1��д��������ڹ�ũҵ�����е���Ҫ����______����дһ����
��2����֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1����ش�
����500�桢200atm��������������һ�ܱ������г���1molN2��3molH2����ַ�Ӧ�ų�������______���������������=����92.4kJ���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=______��
��Ϊ��Ч���������ת���ʣ��˲�ȡ�Ĵ�ʩ��______
A�������¶� B�����ʺϴ������Ե��ʵ����� C������ѹǿ
D������ѹǿ E��ѭ�����úͲ��ϲ��䵪�� F����ʱ�Ƴ���
��3��һ���¶���������̶����ܱ������У���1mol N2��3mol H2��ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ѹǿ�Ƿ�Ӧǰ��0.85������ʱN2��ת����Ϊ______��
��1������ε���Ҫ��;���������ʣ��ʴ�Ϊ���������ʵȣ�
��2������Ϊ���淴Ӧ��������ȫ���е��ף��ų�����������ȫ��Ӧ�٣���1atm��298K�����£�1mol������3mol������ȫ��Ӧ����2mol�������ų�92.4kJ�������÷�ӦΪ���淴Ӧ�������ܽ�����ȫ������Ϊ��Ӧ�¶�Ϊ500�棬���Էų�������С��92.4kJ�� N2��g��+3H2��g��?2NH3��g�����÷�Ӧ��ƽ�ⳣ��K=
��
�ʴ�Ϊ������
��A����Ӧ�Ƿ��ȷ�Ӧ�������¶� ƽ��������У�������ת������ߣ�����ʵ�������в����õ��£���Ϊ�¶ȵͻ�ѧ��Ӧ����������A�����ϣ�
B�����ʺϴ������Ե��ʵ����£��¶�Խ������ת���ʼ��ԣ���B�����ϣ�
C����Ӧ�����������С�ķ�Ӧ������ѹǿ��ƽ��������У�����ת��������C���ϣ�
D������ѹǿ��ƽ��������У���D�����ϣ�
E��ѭ�����úͲ��ϲ��䵪�����������ת���ʣ���E���ϣ�
F����ʱ�Ƴ�����ƽ��������У�����ת��������F���ϣ�
�ʴ�Ϊ��CEF��
��3�����ݻ�ѧƽ�������ʽ��ʽ����õ����赪��ת�����ʵ���Ϊx
N2 +3H2=2NH3
��ʼ����mol�� 1 3 0
ת������mol�� x 3x 2x
ƽ������mol�� 1-x 3-3x 2x
ƽ��������ѹǿ�Ƿ�Ӧǰ��0.85�������ʵ����Ƿ�Ӧǰ��0.85��
��1-x��+��3-3x ��+2x=0.85��4
�õ�x=0.3mol��
������ת����=
��100%=30%
�ʴ�Ϊ��30%��
��2������Ϊ���淴Ӧ��������ȫ���е��ף��ų�����������ȫ��Ӧ�٣���1atm��298K�����£�1mol������3mol������ȫ��Ӧ����2mol�������ų�92.4kJ�������÷�ӦΪ���淴Ӧ�������ܽ�����ȫ������Ϊ��Ӧ�¶�Ϊ500�棬���Էų�������С��92.4kJ�� N2��g��+3H2��g��?2NH3��g�����÷�Ӧ��ƽ�ⳣ��K=
| c2(NH3) |
| c3(H2)c(N2) |
�ʴ�Ϊ������
| c2(NH3) |
| c3(H2)c(N2) |
��A����Ӧ�Ƿ��ȷ�Ӧ�������¶� ƽ��������У�������ת������ߣ�����ʵ�������в����õ��£���Ϊ�¶ȵͻ�ѧ��Ӧ����������A�����ϣ�
B�����ʺϴ������Ե��ʵ����£��¶�Խ������ת���ʼ��ԣ���B�����ϣ�
C����Ӧ�����������С�ķ�Ӧ������ѹǿ��ƽ��������У�����ת��������C���ϣ�
D������ѹǿ��ƽ��������У���D�����ϣ�
E��ѭ�����úͲ��ϲ��䵪�����������ת���ʣ���E���ϣ�
F����ʱ�Ƴ�����ƽ��������У�����ת��������F���ϣ�
�ʴ�Ϊ��CEF��
��3�����ݻ�ѧƽ�������ʽ��ʽ����õ����赪��ת�����ʵ���Ϊx
N2 +3H2=2NH3
��ʼ����mol�� 1 3 0
ת������mol�� x 3x 2x
ƽ������mol�� 1-x 3-3x 2x
ƽ��������ѹǿ�Ƿ�Ӧǰ��0.85�������ʵ����Ƿ�Ӧǰ��0.85��
��1-x��+��3-3x ��+2x=0.85��4
�õ�x=0.3mol��
������ת����=
| 3��0.3 |
| 3 |
�ʴ�Ϊ��30%��

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ