��Ŀ����
���и���Һ�У����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ� ��
| A����0.1 mol��L��1CH3COONa��Һ�У�c(OH��)��c(CH3COOH)��c(H��) |
| B�������£�10 mL 0.01 mol��L��1HCl��Һ��10 mL 0.01 mol��L��1Ba(OH)2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH��12 |
| C��0.1 mol��L��1��KAl(SO4)2��Һ�У�c(SO42-)��c(Al3��)��c(OH��)��c(H��) |
D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ�� ����Һ�����ԣ�����c(Na��)��c(HX)��c(X��)��c(H��)��c(OH��) ����Һ�����ԣ�����c(Na��)��c(HX)��c(X��)��c(H��)��c(OH��) |
A
��

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
 NaOH��Һ�ζ� 20.00mL0.1000 mol��L
NaOH��Һ�ζ� 20.00mL0.1000 mol��L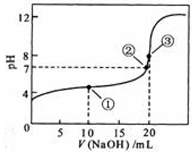

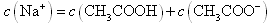


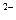 )< c(H2A)
)< c(H2A) c(OH�C) + c(A2�C)
c(OH�C) + c(A2�C)