��Ŀ����
���ѧ��Ӧ����Һ�н��У���������������Һ����Һ�еķ�Ӧ�йء�
(1)ij��ˮ��Ʒ�ղɼ�ʱ���pHΪ4.82�������� ����2Сʱ���ٴβ��pHΪ4.68������������ȷ����
(1)ij��ˮ��Ʒ�ղɼ�ʱ���pHΪ4.82�������� ����2Сʱ���ٴβ��pHΪ4.68������������ȷ����
[ ]
A����ˮ��Ʒ�����С
B����ˮ��Ʒ���û�б仯
C����ˮ��Ʒ�������տ����е�CO2
D����ˮ��Ʒ�е�H2 SO3�������е�����������H2SO4
B����ˮ��Ʒ���û�б仯
C����ˮ��Ʒ�������տ����е�CO2
D����ˮ��Ʒ�е�H2 SO3�������е�����������H2SO4
(2)�ؽ��������ж��ԡ�ʵ�����мס������ַ�Һ������һ�����ԡ���Һ������ʼ��ԣ���Ҫ�ж�����ΪBa2+���罫�ס�������Һ��һ��������ϣ��������Խ��͡��ҷ�Һ�п��ܺ��е�������
[ ]
A��Cu2+��SO42-
B��Cu2+��Cl-
C��K+��SO42-
D��Ag+��NO3-
B��Cu2+��Cl-
C��K+��SO42-
D��Ag+��NO3-
(1)D
(2) A
(2) A

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

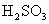 �����������������
�����������������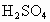
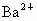 ���ӣ��罫�ס�������Һ��һ��������ϣ��������Խ��ͣ��ҷ�Һ�п��ܺ��е�������
���ӣ��罫�ס�������Һ��һ��������ϣ��������Խ��ͣ��ҷ�Һ�п��ܺ��е�������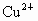 ��
��
 ��
��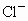
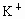 ��
��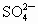
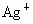 ��
��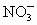
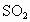 ���壬һ����NaOH��Һ��ˮ���գ��Է���Ⱦ�������ֱ���0.1mol/L��NaOH��Һ��ͬŨ�ȵİ�ˮ������ͬ����β�������ַ�����������Һ�������ϵ��
���壬һ����NaOH��Һ��ˮ���գ��Է���Ⱦ�������ֱ���0.1mol/L��NaOH��Һ��ͬŨ�ȵİ�ˮ������ͬ����β�������ַ�����������Һ�������ϵ�� ��Һ�������˷�����Һ��ʹ
��Һ�������˷�����Һ��ʹ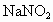 ת��Ϊ�����ʣ��÷�Ӧ���������У�
ת��Ϊ�����ʣ��÷�Ӧ���������У�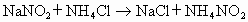
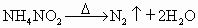
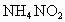 ������������
������������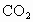
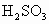 �����������������
�����������������
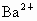 ���ӣ��罫�ס�������Һ��һ��������ϣ��������Խ��ͣ��ҷ�Һ�п��ܺ��е�������
���ӣ��罫�ס�������Һ��һ��������ϣ��������Խ��ͣ��ҷ�Һ�п��ܺ��е�������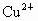 ��
��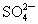
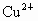 ��
��
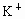 ��
��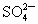
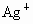 ��
��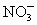
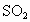 ���壬һ����NaOH��Һ��ˮ���գ��Է���Ⱦ�������ֱ���0.1mol/L��NaOH��Һ��ͬŨ�ȵİ�ˮ������ͬ����β�������ַ�����������Һ�������ϵ��
���壬һ����NaOH��Һ��ˮ���գ��Է���Ⱦ�������ֱ���0.1mol/L��NaOH��Һ��ͬŨ�ȵİ�ˮ������ͬ����β�������ַ�����������Һ�������ϵ�� ��Һ�������˷�����Һ��ʹ
��Һ�������˷�����Һ��ʹ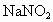 ת��Ϊ�����ʣ��÷�Ӧ���������У�
ת��Ϊ�����ʣ��÷�Ӧ���������У�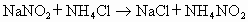

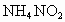 ������������
������������