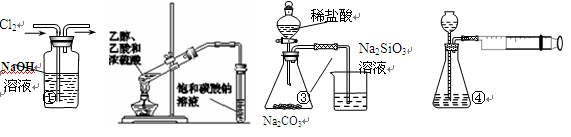
��Ŀ����
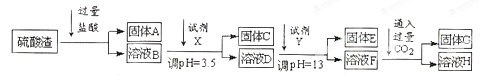
���Ṥҵ�з����ijɷ�ΪSiO2��Fe2O3��Al2O3��MgO�� ij̽����ѧϰС���ͬѧ�������ʵ�鷽�������������н���Ԫ��ת��Ϊ��������������뿪����
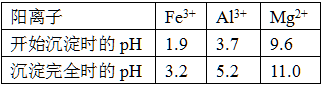
��֪�����£�����������������������ʽ����ʱ��Һ��pH���£�
��1����ҵ���ù���A��ԭ����ȡ�ֹ�Ļ�ѧ����ʽΪ ��
��2��ʵ������11.9mol/L��Ũ��������250mL3.0 mol/L��ϡ���ᣬ���õIJ����������ձ�������������Ͳ�⣬����Ҫ ��
��3�����������е��Լ�Y���ѡ�������е� ��ѡ����ĸ��ţ���
��4��д����ӦF��H�����ӷ���ʽ ��
��5����ҺH�Լ��ԣ�ԭ���� �������ӷ���ʽ�ͱ�Ҫ������˵������
��6��ʵ���ҿ��ô���ʯ��ϡ���ᷴӦ��ȡ���������������CO2���������£�װ��A����CO2���������������Ӹ������ӿڣ�˳��Ϊa�� ��װ��C��Ӧ ʢ�ŵ��Լ�Ϊ ��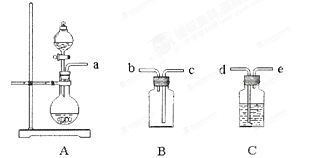

��֪�����£�����������������������ʽ����ʱ��Һ��pH���£�

��1����ҵ���ù���A��ԭ����ȡ�ֹ�Ļ�ѧ����ʽΪ ��
��2��ʵ������11.9mol/L��Ũ��������250mL3.0 mol/L��ϡ���ᣬ���õIJ����������ձ�������������Ͳ�⣬����Ҫ ��
��3�����������е��Լ�Y���ѡ�������е� ��ѡ����ĸ��ţ���
| A��ˮ | B������þ | C����ˮ | D���������� |
��5����ҺH�Լ��ԣ�ԭ���� �������ӷ���ʽ�ͱ�Ҫ������˵������
��6��ʵ���ҿ��ô���ʯ��ϡ���ᷴӦ��ȡ���������������CO2���������£�װ��A����CO2���������������Ӹ������ӿڣ�˳��Ϊa�� ��װ��C��Ӧ ʢ�ŵ��Լ�Ϊ ��

��1��2C��SiO2 Si��2CO��
Si��2CO��
��2��250mL����ƿ����ͷ�ι� (ÿ��1�֣�����ƿ��÷�) ��3��D
��4��AlO2����2H2O��CO2��Al(OH)3����HCO3��(��[Al(OH)4]����CO2��Al(OH)3����HCO3��) ��5��HCO3�� CO32����H+��HCO3����H2O
CO32����H+��HCO3����H2O H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶�
H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶�
��6����d��e��c��b ����NaHCO3��Һ
 Si��2CO��
Si��2CO�� ��2��250mL����ƿ����ͷ�ι� (ÿ��1�֣�����ƿ��÷�) ��3��D
��4��AlO2����2H2O��CO2��Al(OH)3����HCO3��(��[Al(OH)4]����CO2��Al(OH)3����HCO3��) ��5��HCO3��
 CO32����H+��HCO3����H2O
CO32����H+��HCO3����H2O H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶�
H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶���6����d��e��c��b ����NaHCO3��Һ
�����������1���Դֹ����ȡΪ����������ѧ��������ȡ�龳��Ϣ�������������ѧ֪ʶ��֪���ý�̿��ԭ������������ȡ�ֹ衣Ҫ��ѧ�����չ��������ʣ���ȷ��д��̿��������跴Ӧ�ķ���ʽ��
(2������һ�����ʵ���Ũ����Һ����������������ѡ���������������⣬����250mL����ƿ�ͽ�ͷ�ιܡ�Ҫ��ѧ������һ�����ʵ���Ũ����Һ������ʵ�顣
(3)����������Mg2+������������ʽ���������Լ���ѡ���������Ϣ�����̿�֪Ӧ��������������Һ��Mg2+ת����������þ��������ͬʱ��Al3+ת����AlO2������[Al(OH)4]����Ϊ�����������̵档
��4������AlO2������[Al(OH)4]������CO2��Ӧ���ӷ���ʽ����д��Ҫ��ѧ��Ӧ�������CO2��Ӧ�IJ��ﲢ��ȷ��д���ӷ���ʽ��
��5������NaHCO3��Һ�Լ��Ե�ԭ�������ѧ��Ӧ���������ˮ��ƽ�⣬Ҳ���ڵ���ƽ�⣬NaHCO3��Һ֮�����Լ�������ΪHCO3����ˮ��̶ȴ���HCO3���ĵ���̶ȡ�
��6������CO2�����ʵ�����Ʊ������ӡ��ռ�������ѧ�������ʵ�����Ʊ�CO2��ԭ���������������к�������װ�����ô���ʯ��ϡ���᱾����Ӧ�õ���CO2�����л����HCl�������ʣ�Ӧͨ��ʢ�б���NaHCO3��Һ��ϴ��ƿ����ȥ��CO2�����ܶȴ��ڿ�����2���Ʊ��Լ�ˮ��ԭ����Ӧ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��ʵ�����������������ѧ����ѧ������������������ѧ���淶���Ͻ���ʵ����ơ����������Լ���������������������Ҫ��ȷ���������͵�������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д�
�����Ŀ