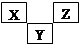��Ŀ����
X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӡ�
��ش��������⣺
(1)Y��Ԫ�����ڱ��е�λ��Ϊ______��
(2)����Ԫ�ص�����������Ӧ��ˮ����������ǿ����______(д��ѧʽ)��
(3)Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������______(д�������������ʵĻ�ѧʽ)��
(4)ZX�ĵ���ʽΪ______��ZX��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ________________________________________________________________________��
��ش��������⣺
(1)Y��Ԫ�����ڱ��е�λ��Ϊ______��
(2)����Ԫ�ص�����������Ӧ��ˮ����������ǿ����______(д��ѧʽ)��
(3)Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������______(д�������������ʵĻ�ѧʽ)��
(4)ZX�ĵ���ʽΪ______��ZX��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ________________________________________________________________________��
(10��) (1)�ڶ����ڵڢ�A��(2)HClO4��(3)Cl2��ClO2��O3(��д����)
(4)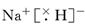 ��NaH��H2O��NaOH��H2��
��NaH��H2O��NaOH��H2��
(4)
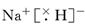 ��NaH��H2O��NaOH��H2��
��NaH��H2O��NaOH��H2�����������X��Zͬ���壬���γ����ӻ�����ZX����˵������Ԫ��Խ���ǵ�IA��Ԫ�أ�����X����Ԫ�ء�Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ���Y����Ԫ�أ�M��SԪ�أ����Z��NaԪ�أ�G����Ԫ�ء�
��1����Ԫ�ص�ԭ��������8����λ�����ڱ��ĵڶ����ڵڢ�A�塣
��2���ǽ�����Խǿ������������Ӧˮ���������Խǿ����Ԫ�صķǽ�������ǿ��������������Ӧ��ˮ����������ǿ����HClO4��
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������Cl2��ClO2��O3�ȡ�
��4���⻯���Ǻ������Ӽ������ӻ��������ʽ��
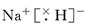 ���⻯�ƺ�ˮ��Ӧ�����������������ƣ���Ӧ�Ļ�ѧ����ʽ��NaH��H2O��NaOH��H2����
���⻯�ƺ�ˮ��Ӧ�����������������ƣ���Ӧ�Ļ�ѧ����ʽ��NaH��H2O��NaOH��H2���������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ