��Ŀ����
18�����ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɣ���1��Al��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2��30Si��ԭ�ӵ�������Ϊ16�� SiO2�ľ�������Ϊԭ�Ӿ��壮
��3��Al3+��Yn-�ĵ�������ͬ��Y������ĸ�Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���HCl��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������CO2��
��5���������������36.0g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0g���壻��Һ�м������NaOH��Һ������õ�21.4g���壻���������Al2O3����������Ϊ25%��
���� ��1����������������Һ��Ӧ����ƫ�����ƺ�������
��2������������=������+�����������������������ݾ�������ʺͳɼ���ʽ�жϾ�������ͣ�SiO2�ľ�������Ϊԭ�Ӿ��壻
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ���Y�ǵ�VIIAԪ�أ��⻯��ķе�������Է����������������������������⻯��е㷴����
��4����ҩƤ�ijɷֺ����ʵ����ʽ����ƶϲ�����ʹ������������������ɷ�Ϊ������̼��
��5��36.0g������Fe2O3��Al2O3��SiO2����������ϡ���ᣬ�������費�����ᷴӦ�����Է���õ�11.0g�Ĺ����Ƕ������裬��Һ�м������NaOH��Һ������õ�21.4g����������������������ԭ���غ������������������ʣ��������������������ٸ�������������ʽ���м��㣮
��� �⣺��1����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����ԭ�ӷ��ϵı���ʽ�����ϽDZ�ʾ�����������½DZ�ʾ������������������=������+�������ɼ����30Si��ԭ�ӵ�������Ϊ��30-14=16��SiO2����Si��O�Թ��ۼ���ɵĿռ�������״�ṹ���۷е�ߣ�Ϊԭ�Ӿ��壬
�ʴ�Ϊ��16��ԭ�Ӿ��壻
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ���Y�ǵ�VIIAԪ�أ��⻯��ķе�������Է������������������HF�к����������HF�ķе����HCl�����Ը����⻯���зе���͵���HCl��
�ʴ�Ϊ��HCl��
��4����ҩƤ�ijɷִ���ʯ��ˮ�ࡢ������֪���ڸ�����ֻ�д���ʯ�ŷֽ����CO2���������ֻ����CO2���壬
�ʴ�Ϊ��CO2��
��5��36.0g������Fe2O3��Al2O3��SiO2����������ϡ���ᣬ�������費�����ᷴӦ�����Է���õ�11.0g�Ĺ����Ƕ������裻
����Һ�м������NaOH��ҺʱAlCl3����NaAlO2��FeCl3����Fe��OH��3���������Է���õ�21.4g��������������������2Fe��OH��3��Fe2O3��������������=$\frac{160��21.4}{214}$g=16g��������������=��36.0-11.0-16��g=9g������������������=$\frac{9g}{36g}$��100%=25%��
�ʴ�Ϊ��25%��
���� ���⿼�����ʵ����ʡ��������͡����ʵĺ����ļ����֪ʶ�������ۺ�֪ʶ�Ŀ��飬��Ŀ�Ѷ��еȣ�ƽʱע�����֪ʶ�����գ�ע������жϾ��������Լ������۷е�ߵ͵ķ�����

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | ����ʱ��������������ֽ���Ӵ� | |
| B�� | �ƾ���ˮ�Ļ�������ͨ�������߷��뿪�� | |
| C�� | ���Թ��еμ�Һ��ʱ����ͷ�ι�Ӧ�����Թ��ڱ� | |
| D�� | KNO3�л���������NaCl����ͨ���ؽᾧʵ�齫���ʳ�ȥ |
| A�� | ���ع��͡���ָ����ˮ����ȡ����֬����Ҳ��һ����Դ��������ʳ�ã�����������ȼ���ͻ������Ʒ��� | |
| B�� | ʯ�������͡����Ͷ����������� | |
| C�� | ��ά�ء�����ϩ�����ڶ��Ǹ߷��ӻ����� | |
| D�� | PM2.5��ָ������ֱ����2.5��10-6m�Ŀ�������ܽ�������ͨ����ˮ���պ����ð�Ĥ�����ķ�������PM2.5��������������� |
Na2O2��s��+CO2��g���TNa2CO3��s��+$\frac{1}{2}$O2��g����H=-226kJ/mol
���������Ȼ�ѧ����ʽ�������ж���ȷ���ǣ�������
| A�� | CO��ȼ����Ϊ283 Kj | |
| B�� | 2 mol CO2��g����2 mol Na2O2��s����Ӧ�ų�452 kJ����ʱ������ת����ԼΪ1.204��1024 | |
| C�� | 2Na2O2��s��+2CO2��s���T2Na2CO3��s��+O2��g����H��-452 kJ/mol | |
| D�� | ��ͼ�ɱ�ʾCO����CO2���ķ�Ӧ���̺�������ϵ |
| A�� | 23gNa��ΪNa+ʱʧȥ�ĵ�����ΪNA | |
| B�� | 18gˮ�����ĵ�����ΪNA | |
| C�� | 8g He�����ķ�����ΪNA | |
| D�� | 16g O2��16g O3������ԭ��������NA |
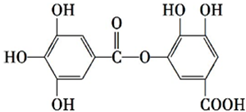
| A�� | �������ԣ�����NaHCO3��Һ��Ӧ | |
| B�� | ��һ�������£�1 mol�������������7 mol NaOH��ȫ��Ӧ | |
| C�� | ��һ�������£�1 mol�����ʿ��Ժ�8 mol H2�����ӳɷ�Ӧ | |
| D�� | �ܷ���ˮ�ⷴӦ��ˮ����������������� |
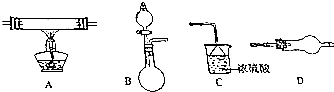 ������ͼ��ʾ��װ�ú�������Ҫ����Ʒ�����÷�Ӧ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O֤���������л�ԭ�ԣ���ش������й����⣮
������ͼ��ʾ��װ�ú�������Ҫ����Ʒ�����÷�Ӧ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O֤���������л�ԭ�ԣ���ش������й����⣮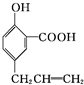 ����
���� ��
��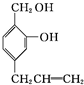 ����
����
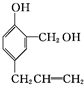
 +4Br2��
+4Br2��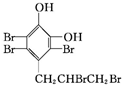 +3HBr��
+3HBr��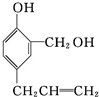 $\stackrel{�Լ�1}{��}$
$\stackrel{�Լ�1}{��}$ $\stackrel{�Լ�2}{��}$
$\stackrel{�Լ�2}{��}$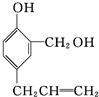 �����Լ�1ΪNa���Լ�2Ϊϡ���ᣨ�����������𰸣���
�����Լ�1ΪNa���Լ�2Ϊϡ���ᣨ�����������𰸣���