��Ŀ����
��7�֣�ÿ��1�֣�A��B��C��DΪͬһ���ڵ�����Ԫ�أ�ԭ����������������֪0.2ĦA�����ַ�Ӧʱ���ڱ���¿�����2.24��������B��������ȿ�����ǿ���ֿ�����ǿ����Һ��C��D�����ӵĵ��Ӳ�ṹ���ԭ����ͬ��C����̬�⻯����C�ĵͼ������ﷴӦ���ֿɵõ�C�ĵ��ʡ��Իش�
��1��A��B��C��D��Ԫ�ط��ŷֱ�Ϊ______��____ �� ��______��
��2���õ���ʽ��ʾA��C�γɻ�����Ĺ��̣�
_________________________________________________��
��3��д��B����������A���������ﷴӦ�����ӷ���ʽ��
_________________________________ ��
��4��д��ʵ�����Ʊ�D�ĵ��ʵĻ�ѧ��Ӧ����ʽ��
__________________________________________��
��ÿ��1�֣���1��Na��Al��S��Cl��2����
��3��Al2O3 + 2OH - = AlO -+ H 2O
��4��MnO2 + 4HCl(Ũ) MnCl2 + Cl2��+ H2O
����:

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
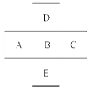
 ��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ� ���������ʼ����ϵ��ͼ��
���������ʼ����ϵ��ͼ��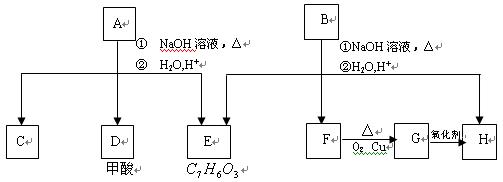
 F��G��H�������л�Ϊͬϵ�����___________��__________��
F��G��H�������л�Ϊͬϵ�����___________��__________��
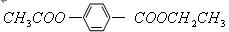
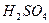 �����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��
�����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��