��Ŀ����
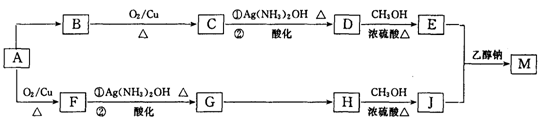
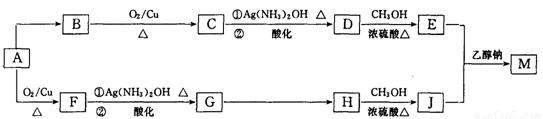
ij���л����ú��ȵ��л���A�ϳ���֯�����̿���������M����ƺϳ�·�����£����ַ�Ӧ�Լ�������δע������
��֪��
�����������D����Է���������90��110֮�䣬����Ԫ�ص���������Ϊ0��615��E��D����Է���������28���˴Ź���������ʾE��������2�ֲ�ͬ��������ԭ�ӣ��������Ϊ3 ��1��

�ش��������⣺
��1��A�����еĺ��������ŵ�������____________��G��H�ķ�Ӧ������____________��
��2��D�ķ���ʽΪ________________��E�Ľṹ��ʽΪ________________��
��3��H��J��Ӧ�Ļ�ѧ����ʽ��______________________��
��4��J��һ�������¿��Ժϳɸ߷��ӻ�����ø߷��ӻ�����ṹ��ʽ��____________��
��5����֪1 mol E��2mol J��Ӧ����l mol M����M�Ľṹ��ʽ��_______________��
��6��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ___________________________________________________��
(1)�ǻ���1�֣�����ȥ��Ӧ��1�֣�
��2��C3H4O4��1�֣���CH3OOCCH2COOCH3��2�֣�
��3�� CH2=CHCOOH + CH3OH CH2=CHCOOCH3
+ H2O��2�֣�
CH2=CHCOOCH3
+ H2O��2�֣�
��4��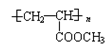 ��2�֣�
��2�֣�
��5��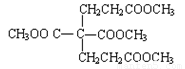 ��2�֣�
��2�֣�
��6��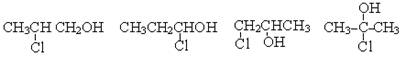 4�֣�
4�֣�
����������

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�