��Ŀ����
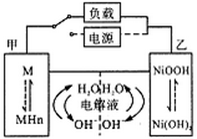
��չ��϶�������ʵʩ���ܼ��ŵ���Ҫ��ʩ֮һ���������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬�Խ�ʡ�ܺġ���϶������ĵ綯��Ŀǰһ��ʹ�õ��������أ������ز������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH�����Һ�������س�ŵ�ԭ���ܷ�ӦʽΪ��H2+2NiOOH 2Ni��OH��2�������йػ�϶��������ж���ȷ���ǣ�
2Ni��OH��2�������йػ�϶��������ж���ȷ���ǣ�
 2Ni��OH��2�������йػ�϶��������ж���ȷ���ǣ�
2Ni��OH��2�������йػ�϶��������ж���ȷ���ǣ�
| A�������»����ʱ���ҵ缫��Χ��Һ��pH����С |
| B�������»����ʱ����Һ�е�K+��缫Ǩ�� |
| C����ɲ��������ʱ���ҵ缫���� |
D����ɲ��������ʱ���缫�ĵ缫��ӦʽΪ��2H2O+2e- H2��+2OH- H2��+2OH- |
D
����������������»����ʱ���綯���ṩ�ƶ���Ϊԭ��أ��缫Ϊ��������ӦʽΪH2+2OH--2e-=2H2O���Ҽ�Ϊ��������ӦʽΪ2NiOOH+2H2O+2e-
 2Ni(OH)2+2OH-��A���ҵ缫��Χ��Һ��pH��������B�������»����ʱ����Һ�е�K+�������ҵ缫Ǩ�ƣ�����C����ɲ��������ʱ����Ϊ������Ni(OH)2��ΪNiOOH��������С������D����ȷ��
2Ni(OH)2+2OH-��A���ҵ缫��Χ��Һ��pH��������B�������»����ʱ����Һ�е�K+�������ҵ缫Ǩ�ƣ�����C����ɲ��������ʱ����Ϊ������Ni(OH)2��ΪNiOOH��������С������D����ȷ��
��ϰ��ϵ�д�
�����Ŀ
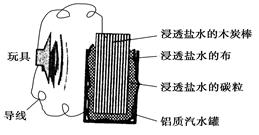

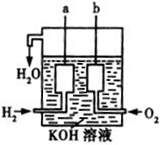


 2CH3CHO��2H2O
2CH3CHO��2H2O