��Ŀ����
��8�֣���֪��
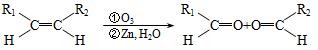
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
�١�CH3 ��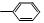 �ۡ�CH�TCH2
�ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
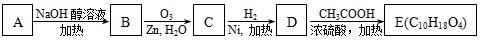
�ṹ��������E�����к�����������û��֧����
��д������ת���ķ�Ӧ���ͣ�
A��B_______________��D��E_________________��
��A��E�Ľṹ��ʽ�ֱ�Ϊ ��
��д��A��B�Ļ�ѧ����ʽ_____________ ____
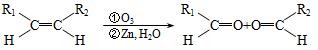
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
�١�CH3 ��
 �ۡ�CH�TCH2
�ۡ�CH�TCH2��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
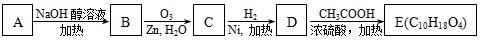
�ṹ��������E�����к�����������û��֧����
��д������ת���ķ�Ӧ���ͣ�
A��B_______________��D��E_________________��
��A��E�Ľṹ��ʽ�ֱ�Ϊ ��
��д��A��B�Ļ�ѧ����ʽ_____________ ____
��ÿ��1�֣���8�֣���1��ȩ�����٢� ��2���ڢ�
��3������ȥ ��Ӧ��������Ӧ
��Ӧ��������Ӧ
��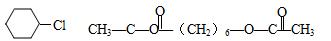
����
��3������ȥ
 ��Ӧ��������Ӧ
��Ӧ��������Ӧ��
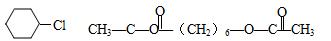
����
��

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ
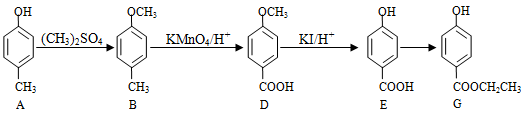
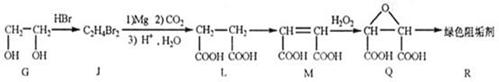
 ��ɫ��ԭ���� ��������A��ˮ�е��ܽ�ȱȱ��ӵ� �����С������
��ɫ��ԭ���� ��������A��ˮ�е��ܽ�ȱȱ��ӵ� �����С������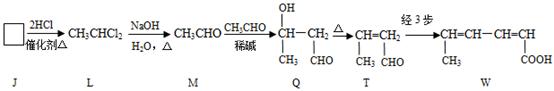
 ��
�� Q
Q 20�֣�
20�֣� ���л���Ľṹ��ʽ
���л���Ľṹ��ʽ
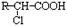 ��
��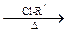 R-O-R�� (R-��R��-��������)��
R-O-R�� (R-��R��-��������)��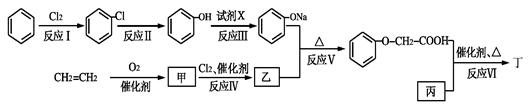
 CH3CHBrCH2Br ��CH3CH2OH
CH3CHBrCH2Br ��CH3CH2OH  CH2=CH2+H2O
CH2=CH2+H2O
 RCOCl(����)��RCOCl + NH3��RCONH2 + HCl��
RCOCl(����)��RCOCl + NH3��RCONH2 + HCl��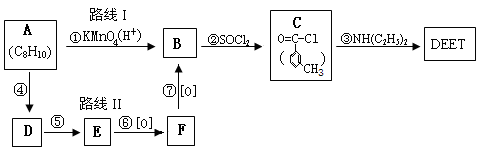
 Һ�� D��������
Һ�� D��������

 _ˮ���Ƶá�(����ࡱ������֬�������ʡ�)
_ˮ���Ƶá�(����ࡱ������֬�������ʡ�) ___
___
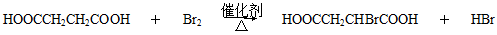
 ��Ӧʹ��ˮ��ɫ����
��Ӧʹ��ˮ��ɫ����