��Ŀ����
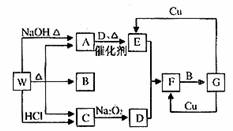
W��һ���Σ�����������ת����ϵ�����ֲ�������ȥ��������A��C��D����ɫ���塣
��ش��������⣺
��1��A�ĵ���ʽ�� ��
��2��������з�Ӧ�ķ���ʽ�������ӷ�Ӧ��ֻд���ӷ���ʽ��
A��E�� ��
G��E�� ��
��3��E��Dǡ����ȫ��Ӧʱ���õ���F�������ǻ���ԭ���ǣ���ϻ�ѧ����ʽ�ش�
��
��4�������ҹ���һ��̽�������϶�һ�ŵĻ�����ҹ��Լ�����ij������ż�������ʹ�õ��ƽ������������ͻ�ԭ����ɡ�F�е���ɫ���ʾ�Һ������Ϊ�������ż��һ�����ƽ����е�����������ԭ������Է�������Ϊ60��̼���⡢������Ԫ�ص�������Ϊ6:2:7��Һ̬�л���û�ԭ���Ļ�ѧʽ�� ����5g��ԭ������������ȫȼ��ʱ��������B��C���Լ�һ�ֲ������ѭ�������壬���ų�212.5kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1��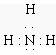
��2��4NH3+5O2![]() 4NO+6H2O��3Cu+8H++2NO
4NO+6H2O��3Cu+8H++2NO![]() =3Cu2++2NO��+4H2O
=3Cu2++2NO��+4H2O
��3��2NO2![]() N2O4����������������������֮����ڻ�ѧƽ�⣬�����������������������档
N2O4����������������������֮����ڻ�ѧƽ�⣬�����������������������档
��4��C2H8N2��C2H8N2(l)+2N2O4��l��=2CO2(g)+4H2O(g)+3N2(g)����H=��2550kJ/mol

