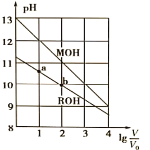
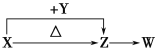��Ŀ����
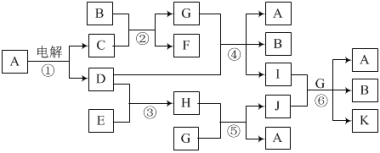
����Ŀ����һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�����ͼ��ʾ��ʵ��װ�ã�ͨ��������Ӧ���ɵ�CO2��H2O����������ȷ���û�����и���ֵ�����������

��1��ʵ�鲽�裺���г�װ��δ������
�ٰ�ͼ��װ��ʵ��װ�ú����Ƚ��еIJ�����_____��
�ڳ�ȡ��Ʒ���������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC��װ��ʯ�ҵ�U�ι�D��������
�۴���K1��K2���ر�K3������������������ӣ���Ŀ����____��
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣
�ݴ���K1������������������ӣ�Ȼ�����װ�ã��ٴγ���ϴ��ƿC��U�ι�D��������
��������ʵ�鷽������ش��������⡣
��2������Ӧ����������ᵼ�²�����NaCl��������___��������ƫ��������ƫС��������Ӱ��������
��3��E���������ʢ�ŵ�ҩƷ��____����������____�����û�и�װ�ã��ᵼ�²�����NaHCO3����������___������ƫ��������ƫС��������Ӱ��������
��4������Ʒ����Ϊwg����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1g��m2g����������Na2CO3��10H2O����������Ϊ____���ú�w��m1��m2�Ĵ���ʽ��ʾ����
���𰸡����װ�������� ��ȥװ���е�ˮ�����Ͷ�����̼ ƫ�� ��ʯ�� ��ֹ�����е�CO2��ˮ��������D��Ӱ��ⶨ��� ƫ�� 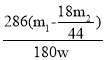 ��100%
��100%
��������
���������Ȼ����H2O��g����CO2�����壬Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ��������CO2����D�����أ�NaHCO3�ֽ������CO2�������������NaHCO3��������C�����أ�Na2CO3��10H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O�������������Na2CO3��10H2O���������Ӷ����NaCl����������Ӧ��ʵ��ǰ�뷨�ϳ�װ���еĿ������ؼ�����Ӧ�Ǹ�B�еĿ���������K1��K2���ر�K3���ͳ�Ϊ�����Ĺؼ�������ͨ������Ϊ�˸ϳ�Ч�����ã�E�м�ʯ�ҿɷ�ֹ�������е�H2O��g����CO2����װ��DӰ��ʵ��Ч��.
��1�������巢��װ����Ҫ���װ�������ԣ�
��װ�����п���������ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ����ʵ��ǰҪͨ�����������װ���к���ˮ�����Ͷ�����̼�������������IJ��������ǣ�����K1��K2���رջ���K3��
��2�������ȷ�Ӧ�������������ˮ���������Ͷ�����̼�����ⶨ������С��
��3��E��������Ǽ�ʯ�ң���ֹ������ˮ�����Ͷ�����̼����װ��DӰ��ⶨ��������ʵ����û�и�װ��Dװ���вⶨ������̼��������
��4������Dװ�������������������յĶ�����̼����������̼�����Ʒֽ�ʱ���ɵĶ�����̼��ˮ�����Ĺ�ϵʽ����̼���������ɵ�ˮ�������ܵ�ˮ������ȥ̼���������ɵ�ˮ��������ʮˮ̼���Ʒֽ����ɵ�ˮ����������ʮˮ̼���Ʒֽ����ɵ�ˮ������ʮˮ̼���ƵĹ�ϵʽ����ʮˮ̼���Ƶ��������Ӷ�����������������
��1������ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ��װ�ã��������ɵ�ˮ����������Ӧ���ȼ���װ�õ������ԡ�
��װ�����п���������ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ������K1��K2���رջ���K3��ʵ��ǰҪͨ�����������װ���к���ˮ�����Ͷ�����̼��������
��2�������ȷ�Ӧ�������������ˮ���������Ͷ�����̼�����ⶨ������С��̼���������ݶ�����̼���㣬��Na2CO3��10H2O�IJⶨ�Ǹ�������ˮ������������ģ�����Na2CO3��10H2O�ĺ�����ƫС�Բⶨ�����Ӱ����NaClƫ��
��3���������ʢ�ŵ��Ǽ�ʯ�ң���ʯ�������տ����е�ˮ�����Ͷ�����̼�����Ը���ܵ������Ƿ�ֹ�����е�CO2��ˮ��������Ӱ��ⶨ���������ȥEװ�ã���ⶨ��̼�����Ƶ�����ƫ��
��4��Dװ�������ӵ�����Ϊ������̼��������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
2NaHCO3![]() Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
18g 44g
x m2g
x=9m2/22 g��
װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�ʮˮ̼���Ʒֽ����ɵģ�ʮˮ̼���Ʒֽ����ɵ�ˮ����������=m1g-9m2/22 g=(m1-9m2/22 )g��
��ʮˮ̼���Ƶ�����Ϊy��
Na2CO3��10H2O![]() Na2CO3+10H2O
Na2CO3+10H2O
286g 180g
y (m1-9m2/22 )g
y=286(m1-9m2/22 )/180g
����ʮˮ̼���Ƶ���������=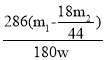 ��100%
��100%