��Ŀ����
��Na+�����ʵ���Ũ��Ϊ0.5mol?L-1��ij������Һ�У������ܺ����������ʾ�����������ӣ�
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO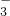 ��CO ��CO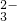 ��SiO ��SiO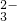 ��SO ��SO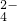 |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�м�������ϡ���� | ���ɰ�ɫ�������ڱ�״���·ų�0.56L���� |
| �� | �����в����Ļ��Һ���ˣ�������ϴ�ӡ����������أ��������ù�������� | ��������Ϊ2.4g |
| �� | ��������õ���Һ�еμ�BaCl2��Һ | ���������� |
��1����ʵ���ȷ��һ�������ڵ�������______��
��2��ʵ��������ɳ��������ӷ���ʽΪ______��
��3��ͨ��ʵ���ͱ�Ҫ���㣬��д���������ӵ����ʵ���Ũ�ȣ��ܼ�����������д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ������������
| ������ | NO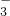 | CO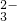 | SiO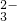 | SO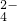 |
| c/mol?L-1 | ______ | ______ | ______ | ______ |
�⣺������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������森��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ =0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ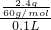 =0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
�ʴ�Ϊ��Ag+��Mg2+��Ba2+��
��2��ʵ��������ɳ��������ӷ���ʽΪ��SiO32-+2H+=H2SiO3����
�ʴ�Ϊ��SiO32-+2H+=H2SiO3����
��3��ͨ���������������֪��
��4�����ݵ���غ�2c��CO32-+��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L��
�ʴ�Ϊ�����ڣ���Ũ������Ϊ0.8mol/L��
������������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������森��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ =0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ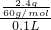 =0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
���������⿼�����ӹ���ȣ��Ѷ��еȣ�ע���������ӷ�Ӧ�����ݵ���غ��ж�K+�Ƿ���ڣ��DZ�����ѵ㡢�״��㣬�Ѷ��еȣ�
 =0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ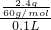 =0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ���1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
�ʴ�Ϊ��Ag+��Mg2+��Ba2+��
��2��ʵ��������ɳ��������ӷ���ʽΪ��SiO32-+2H+=H2SiO3����
�ʴ�Ϊ��SiO32-+2H+=H2SiO3����
��3��ͨ���������������֪��
| ������ | NO3- | CO32- | SiO32- | SO42- |
| c/mol?L-1 | �� | 0.25mol/L | 0.4mol/L | 0 |
�ʴ�Ϊ�����ڣ���Ũ������Ϊ0.8mol/L��
������������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������森��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ
 =0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ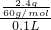 =0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ����������⿼�����ӹ���ȣ��Ѷ��еȣ�ע���������ӷ�Ӧ�����ݵ���غ��ж�K+�Ƿ���ڣ��DZ�����ѵ㡢�״��㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ