��Ŀ����
�ۺ��Ȼ�������[A12��OH��nCl6-n��?H2O]m�ǽ���AlCl3��Al��OH��3֮���һ��ˮ�������߷��Ӿۺ�����Ʊ�ԭ����Ҫ�����ӹ���ҵ�ķ���--���ң�����Ҫ��Al2O3��Al������SiO2�����ʣ��ۺ��Ȼ������������������£�
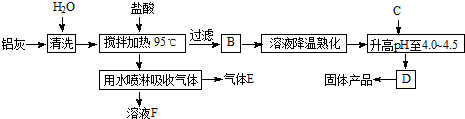
��1����Ӧ�и���ƷE�� ���û�ѧʽ��ʾ����
��2������pH��4.0��4.5��Ŀ���� ��
��3�����������п�ѭ��ʹ�õ������� ���û�ѧʽ��ʾ����
��4��Ϊʹ�õ��ľ���ϴ���������������ʹpH���ߵ�c���ʿ�ѡ�� �����ţ���
a��NaOH b��Al c����ˮ d��A12O3 e��NaAlO2
��5��Ϊ�ⶨ[A12��OH��nCl6-n��?H2O]m�е�nֵ����������ʵ�飺
�ٳ�ȡag���壬�Ƴɷ�ĩ���������������ٱ仯ʱ���õ�bg���壮�˹��̿����õ��� ���������� ��
a�������� b������ c���в� d���Թ�
����ȡa g���壬�������²����� ��
��A�Լ��ܽ��������AgNO3��Һ������c����ɡ�����Ϊc g���壮�Լ�AΪ ������CΪ �� ����������ƣ���n= ���ú�a��b��c�Ĵ���ʽ��ʾ����

��1����Ӧ�и���ƷE��
��2������pH��4.0��4.5��Ŀ����
��3�����������п�ѭ��ʹ�õ�������
��4��Ϊʹ�õ��ľ���ϴ���������������ʹpH���ߵ�c���ʿ�ѡ��
a��NaOH b��Al c����ˮ d��A12O3 e��NaAlO2
��5��Ϊ�ⶨ[A12��OH��nCl6-n��?H2O]m�е�nֵ����������ʵ�飺
�ٳ�ȡag���壬�Ƴɷ�ĩ���������������ٱ仯ʱ���õ�bg���壮�˹��̿����õ��� ����������
a�������� b������ c���в� d���Թ�
����ȡa g���壬�������²�����
��A�Լ��ܽ��������AgNO3��Һ������c����ɡ�����Ϊc g���壮�Լ�AΪ
��������1��������Ȳ������̣��������ᣬ�������ᷴӦ�����������ɣ����ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq����ʣ������Ϊ������
��2����Һ��pHֵ���ٽ�������ˮ�⣬���þ���������
��3��95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq��������ѭ��ʹ�ã�
��4������c���ǵ���pHֵ���ٽ�������ˮ�⣬���������������ʣ����������ͼ�е�B�л����ٻ�����������HCl����Ϊ���Ȳ��ܱ�֤���е�HClȫ���ӷ���
��5�����ݸ�����ѧ������ʹ�÷�������;���ش�
��6�����������Ӽ���ķ����Լ��õ����Լ�֪ʶ���ش�
��2����Һ��pHֵ���ٽ�������ˮ�⣬���þ���������
��3��95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq��������ѭ��ʹ�ã�
��4������c���ǵ���pHֵ���ٽ�������ˮ�⣬���������������ʣ����������ͼ�е�B�л����ٻ�����������HCl����Ϊ���Ȳ��ܱ�֤���е�HClȫ���ӷ���
��5�����ݸ�����ѧ������ʹ�÷�������;���ش�
��6�����������Ӽ���ķ����Լ��õ����Լ�֪ʶ���ش�
����⣺��1��������Ȳ������̣��������ᣬ�������ᷴӦ�����������ɣ����ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq����ʣ������Ϊ��������Ӧ�и���ƷaΪH2���ʴ�Ϊ��H2��
��2��������ˮ�⣬Al3++3H2O?Al��OH��3+3H+������������Ũ�ȴٽ�������ˮ�⣬�����ھۺ��Ȼ��������������ʴ�Ϊ���ٽ�AlCl3ˮ�⣬ʹ����������
��3��95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq�����ɽ���ѭ��ʹ�ã�
�ʴ�Ϊ��HCl��
��4�����������ƺͰ�ˮ����pHֵ���������µ����ʣ����������Ӻ�笠����ӣ����Կ��Լ���Al�����������д������������ǹ��壬���˿��Թ��˵��ģ����Կ���ʹ�õ��ľ���ϴ������ʴ�Ϊ��bd��
��5���ٳ�ȡ������Ƴɷ�ĩ��Ӧ���õ��в����������������ٱ仯ʱ��Ӧ�������������ȣ��ʴ�Ϊ��bc��
�ڽ�[A12��OH��nCl6-n��?H2O]m�ܽ����������ữ�����������Խ�������ȫ���������Ƴ���������������Ԫ���غ���Եõ������ӵ�������Ϊ�����õ���cg������AgCl����ȡag���壬�Ƴɷ�ĩ���������������ٱ仯ʱ���õ�bg���壬��A12��OH��nCl6-n��������b����ClԪ���غ㣬��
=
��(6-n)�����n=
���ʴ�Ϊ��
��
��2��������ˮ�⣬Al3++3H2O?Al��OH��3+3H+������������Ũ�ȴٽ�������ˮ�⣬�����ھۺ��Ȼ��������������ʴ�Ϊ���ٽ�AlCl3ˮ�⣬ʹ����������
��3��95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq�����ɽ���ѭ��ʹ�ã�
�ʴ�Ϊ��HCl��
��4�����������ƺͰ�ˮ����pHֵ���������µ����ʣ����������Ӻ�笠����ӣ����Կ��Լ���Al�����������д������������ǹ��壬���˿��Թ��˵��ģ����Կ���ʹ�õ��ľ���ϴ������ʴ�Ϊ��bd��
��5���ٳ�ȡ������Ƴɷ�ĩ��Ӧ���õ��в����������������ٱ仯ʱ��Ӧ�������������ȣ��ʴ�Ϊ��bc��
�ڽ�[A12��OH��nCl6-n��?H2O]m�ܽ����������ữ�����������Խ�������ȫ���������Ƴ���������������Ԫ���غ���Եõ������ӵ�������Ϊ�����õ���cg������AgCl����ȡag���壬�Ƴɷ�ĩ���������������ٱ仯ʱ���õ�bg���壬��A12��OH��nCl6-n��������b����ClԪ���غ㣬��
| c |
| 143.5 |
| b |
| 54+17n+35.5(6-n) |
| 861b-102c |
| 143.5b |
| 861b-102c |
| 143.5b |
�����������Ծۺ��Ȼ���������Ʊ�Ϊ���壬���������仯�������ʡ����ӷ���ʽ���Թ������̵����⡢���ӵȣ��Ѷȴؼ����ڶԹ������̵������֪ʶ��Ǩ�����ã�

��ϰ��ϵ�д�
�����Ŀ



