��Ŀ����
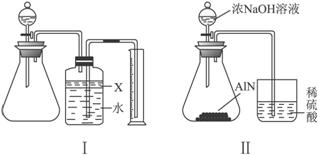
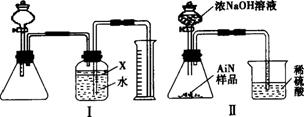
������(AlN)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij��������Ʒ�п��ܺ���̼���������е�һ�����ʣ�����ͼ����ʾ��װ�������м��飬ʹ��������Ʒ��NaOH��Һ��ӦAlN+NaOH+H2O
ͼ��
(1)ʵ���йز���Ϊ��
a.��Բ����ƿ�з���AlN��Ʒw g��������ƿ�м���ˮ��XҺ��
b.�ӷ�Һ©����Բ����ƿ�м���һ������Ĺ�����ŨNaOH��Һ
c.����װ�õ�������
d.��ȡ�ռ���ˮ�����
��ȷ�IJ���˳��Ϊ______________________��
(2)��ʵ���м��װ�������Եķ�����________________________��
(3)���ƿ�е��Լ�X��ѡ��___________(��ѡ��ı��)��
A.���� B.�ƾ� C.ֲ���� D.CCl4
(4)ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������________________��
(5)ʵ�������Һ©���е�NaOH��Һ�Ѿ�ȫ��������ƿ����Ͳ���ռ���ˮ�����Ϊa L������ʱ��ʵ������Ϊ��״��������Ʒ�е�AlN����������Ϊ___________(AlN��ʽ��Ϊ41)����һ�ⶨ�����ʵ��ֵƫ�ߣ�����Ϊ���ܵ�ԭ����__________________________��
(6)���˽������ͼ��װ�ý���ͬ��ʵ�飬��ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�����ĸĽ��Ƿ���У�________________(����С������С�)��������________________________��
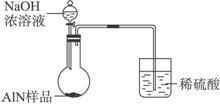
ͼ��
(1)c��a��b��d
(2)�رշ�Һ©������������Ͳ�м�����ˮ��û���ܿڣ���Բ����ƿ����Ͳ�в������ݣ��ָ�������ʱ�������γ�һ��Һ��
(3)C (4)̼
(5)![]() ��100% ����Ĺ�������������Һռ��һ���������ʹ�ų���ˮ�����ƫ��
��100% ����Ĺ�������������Һռ��һ���������ʹ�ų���ˮ�����ƫ��
(6)������ �������ױ��������գ�������������
����:�������⣬��ʵ��Ϊһ����ʵ�飬��˰�װ��ͼ����װ�ú�����Ҫ����װ�õ������ԣ��䷽���ǣ��رշ�Һ©������������Ͳ�м�����ˮ��û���ܿڣ�ʹװ���ܱգ�����Բ����ƿ��ʹװ��������������ͣ�����Ͳ�в������ݣ��ָ�������ʱ�������γ�һ��ˮ���������������á�����NH3��������ˮ����ˣ���NH3��ˮʱ���������Լ�X��NH3��H2O���������ƿ�е��Լ�X����߱������������ٲ���NH3��H2O��Ӧ�����ܽ�NH3��������ˮ�����ܶȱ�H2OС�������Լ�X��ѡ��ֲ���͡�ʵ�����������ƿ�л��й��壬����Ʒ�к��е�������̼����ΪAlN��Al2O3����NaOH��Һ��Ӧ���ܽ���NaOH��Һ�С�
��AlN+NaOH+H2O![]() NaAlO2+NH3��
NaAlO2+NH3��
![]() a L
a L
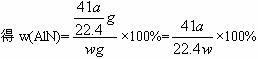
�ⶨ�����ʵ��ֵƫ�ߣ�����Ϊ����Ĺ���NaOH��Һռ��һ���������ʹ�ų���ˮ�����ƫ��aֵƫ��

 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д�