��Ŀ����
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
(1)���������У�SO2����������SO3�� 2SO2(g)��O2(g)![]() 2SO3(g)��ij�¶��£�SO2��ƽ��ת����()����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g)��ij�¶��£�SO2��ƽ��ת����()����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������ͼʾ�ش��������⣺
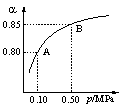
�ٽ�2.0 mol SO2��1.0 mol O2����10 L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10 MPa���÷�Ӧ��ƽ�ⳣ������____________��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A)_______K(B)(���������������=��)��
(2)��CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)��4NO2(g) = 4NO(g)��CO2(g)��2H2O(g)��DH =��574 kJ/mol
CH4(g)��4NO(g) = 2N2(g)��CO2(g)��2H2O(g)��DH =��1160 kJ/mol
���ñ�״����4.48 L CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ_____________(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ_________kJ��
(3)�������ײ�����ȱλ������(MFe2Ox��3��x��4��M = Mn��Co��Zn��Ni)��������(MFe2O4)�����»�ԭ���ã������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
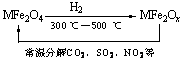
��д��MFe2Ox�ֽ�SO2�Ļ�ѧ����ʽ______________________________________(������ƽ)��
(1)��800 L?mol��1���� =�� (2)1.60NA(��1.6NA)��173.4�� (3)MFe2Ox��SO2![]() MFe2O4��S��
MFe2O4��S��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д� ��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��



 ��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
 ��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��